Defining Concentration
The concentration of a solution is a measure of the amount of solute dissolved in a specific volume of solvent, which is typically water in A-Level Chemistry. The standard unit for concentration is moles per cubic decimetre (mol dm⁻³).
It is important to remember the conversion between cubic centimetres (cm³) and cubic decimetres (dm³):
- 1 dm³ = 1000 cm³
A solution with a high amount of solute is described as concentrated, while one with a low amount of solute is described as dilute. The relationship between concentration, moles, and volume is given by the formula:
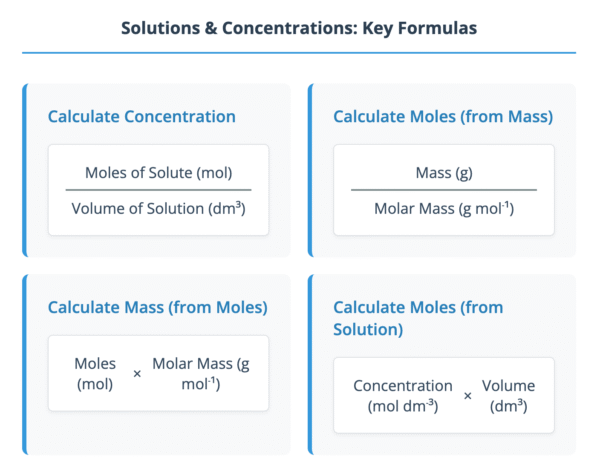
Concentration (mol dm⁻³) = Moles of solute (mol) / Volume of solution (dm³)
Calculating Concentration from Mass
To find the concentration of a solution when you know the mass of the solute and the total volume of the solution, a two-step calculation is required.
- Calculate the moles of the solute by dividing its mass in grams by its molar mass (Mr).
Moles = Mass (g) / Molar Mass (g mol⁻¹) - Convert the volume of the solution from cm³ to dm³ by dividing by 1000.
- Use the concentration formula to determine the concentration in mol dm⁻³.
Example Calculation
Calculate the concentration of a solution containing 4.0 g of sodium hydroxide (NaOH) in 250 cm³ of solution. (Mr of NaOH = 40.0 g mol⁻¹)
Step 1: Calculate moles of NaOH.
Moles = 4.0 g / 40.0 g mol⁻¹ = 0.10 mol
Step 2: Convert volume to dm³.
Volume = 250 cm³ / 1000 = 0.250 dm³
Step 3: Calculate concentration.
Concentration = 0.10 mol / 0.250 dm³ = 0.40 mol dm⁻³
Calculating Mass from Concentration
It is often necessary to calculate the mass of a solute present in a solution of a known concentration and volume. This involves rearranging the concentration formula.
- Calculate the moles of solute by multiplying the concentration by the volume in dm³.
Moles = Concentration (mol dm⁻³) × Volume (dm³) - Calculate the mass of the solute by multiplying the moles by the molar mass.
Mass (g) = Moles (mol) × Molar Mass (g mol⁻¹)
Example Calculation
Calculate the mass of anhydrous copper(II) sulfate (CuSO₄) in 50 cm³ of a 0.20 mol dm⁻³ solution. (Mr of CuSO₄ = 159.6 g mol⁻¹)
Step 1: Calculate moles of CuSO₄.
Volume = 50 cm³ / 1000 = 0.050 dm³
Moles = 0.20 mol dm⁻³ × 0.050 dm³ = 0.010 mol
Step 2: Calculate mass of CuSO₄.
Mass = 0.010 mol × 159.6 g mol⁻¹ = 1.6 g (to 2 significant figures)
Titration Calculations
Titration is a practical technique used to determine the unknown concentration of a solution by reacting it with a solution of known concentration. Calculations based on titration data follow a clear, logical sequence.
- Calculate the moles of the reactant with the known concentration and volume (the standard solution).
Moles = Concentration × Volume (in dm³) - Use the mole ratio (stoichiometry) from the balanced chemical equation to determine the number of moles of the other reactant.
- Calculate the unknown concentration using the moles determined in step 2 and the volume of the solution used in the titration.
Concentration = Moles / Volume (in dm³)
Example Calculation
In a titration, 25.0 cm³ of sodium hydroxide solution is exactly neutralised by 15.00 cm³ of 0.200 mol dm⁻³ sulfuric acid (H₂SO₄). Calculate the concentration of the sodium hydroxide solution.
The balanced equation is: 2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
Step 1: Calculate moles of H₂SO₄.
Volume of H₂SO₄ = 15.00 cm³ / 1000 = 0.01500 dm³
Moles of H₂SO₄ = 0.200 mol dm⁻³ × 0.01500 dm³ = 0.00300 mol
Step 2: Use the mole ratio to find moles of NaOH.
From the equation, the ratio of NaOH to H₂SO₄ is 2:1.
Moles of NaOH = 0.00300 mol × 2 = 0.00600 mol
Step 3: Calculate the concentration of NaOH.
Volume of NaOH = 25.0 cm³ / 1000 = 0.0250 dm³
Concentration of NaOH = 0.00600 mol / 0.0250 dm³ = 0.240 mol dm⁻³
