Water of Crystallisation
Some crystalline compounds incorporate a fixed number of water molecules into their structural lattice. This water is referred to as the water of crystallisation.
Key Definitions
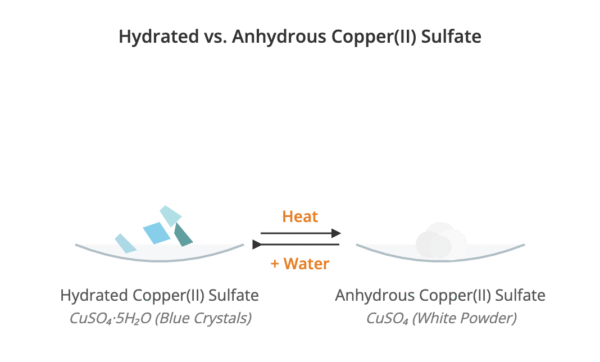
A hydrated compound is a substance that contains water of crystallisation within its structure. A well-known example is hydrated copper(II) sulfate, CuSO₄·5H₂O, which is blue.
An anhydrous compound is a substance that does not contain water of crystallisation. For example, anhydrous copper(II) sulfate, CuSO₄, is a white powder.
A single compound can have different degrees of hydration. For instance, cobalt(II) chloride exists as both cobalt(II) chloride-6-water (CoCl₂·6H₂O) and cobalt(II) chloride-2-water (CoCl₂·2H₂O).
When writing formulae for hydrated compounds, a dot is used to separate the main formula from the water of crystallisation.
Comparison of Hydrated and Anhydrous Compounds
| Feature | Hydrated Compound | Anhydrous Compound |
| Definition | Contains a fixed ratio of water molecules (water of crystallisation) within its crystal structure. | Does not contain water of crystallisation. |
| Appearance (Example) | Blue crystals (CuSO₄·5H₂O) | White powder (CuSO₄) |
| Formula (Example) | CuSO₄·5H₂O | CuSO₄ |
| Formation | Formed when water is added to an anhydrous compound. | Formed when a hydrated compound is heated. |
Reversible Reactions
The process of hydration and dehydration is reversible. A hydrated compound can be formed by adding water to an anhydrous compound, and this reaction can be reversed by heating.
- Hydration: An anhydrous compound becomes a hydrated compound upon the addition of water.
CuSO₄(s) + 5H₂O(l) → CuSO₄·5H₂O(s) - Dehydration: Heating a hydrated compound removes the water of crystallisation, leaving the anhydrous compound.
CuSO₄·5H₂O(s) → CuSO₄(s) + 5H₂O(g)
Calculating Relative Formula Mass
To calculate the relative formula mass (Mᵣ) of a hydrated salt, the mass of the anhydrous part and the mass of the water of crystallisation are calculated separately and then added together.
For example, to find the Mᵣ of hydrated magnesium nitrate-6-water, Mg(NO₃)₂·6H₂O:
- Calculate the Mᵣ of Mg(NO₃)₂: 24.3 + 2 × (14.0 + (3 × 16.0)) = 148.3
- Calculate the mass of 6H₂O: 6 × ((2 × 1.0) + 16.0) = 108.0
- Add the two values: 148.3 + 108.0 = 256.3
