Ionic Bonding
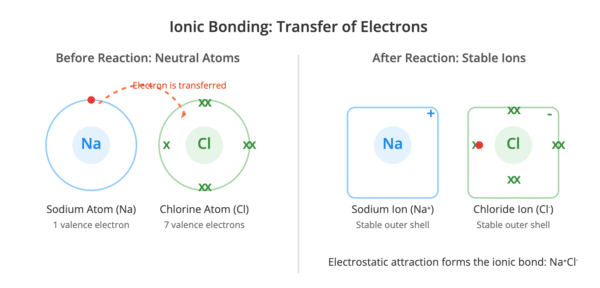
Ionic bonding is the electrostatic force of attraction between positively charged ions (cations) and negatively charged ions (anions), which are arranged in a giant crystal lattice. This type of bonding typically occurs between metals and non-metals.
Metal atoms lose their outer shell electrons to form stable, positively charged ions, achieving the electronic configuration of a noble gas. Non-metal atoms gain these electrons to form stable, negatively charged ions, also achieving a noble gas configuration. The transfer of electrons is a key characteristic of ionic bond formation.
For example, in sodium chloride (NaCl), a sodium atom transfers its single outer electron to a chlorine atom. This results in a sodium ion (Na⁺) and a chloride ion (Cl⁻).
In magnesium oxide (MgO), a magnesium atom transfers its two outer electrons to an oxygen atom, forming an Mg²⁺ ion and an O²⁻ ion. In calcium fluoride (CaF₂), one calcium atom transfers one electron to each of two fluorine atoms, creating one Ca²⁺ ion and two F⁻ ions.
Covalent Bonding
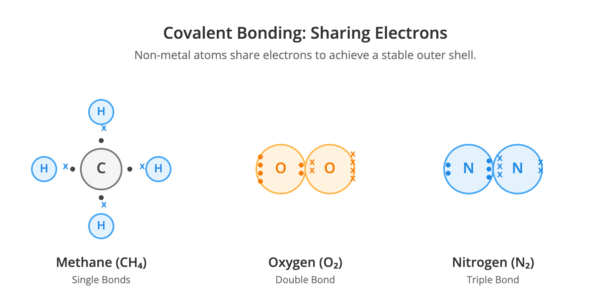
A covalent bond is formed by the electrostatic attraction between the positive nuclei of two atoms and a shared pair of electrons. This type of bonding occurs between non-metal atoms.
Single, Double, and Triple Covalent Bonds
Atoms share electrons to achieve a stable outer shell, similar to that of a noble gas.
- A single covalent bond consists of one shared pair of electrons (e.g., in H₂, CH₄, H₂O, NH₃).
- A double covalent bond involves two shared pairs of electrons (e.g., in O₂, CO₂).
- A triple covalent bond is formed by sharing three pairs of electrons (e.g., in N₂).
Double bonds are generally shorter and stronger than single bonds between the same two elements.
Exceptions to the Octet Rule
Some molecules do not follow the standard octet rule for the central atom.
- Electron-deficient molecules, such as boron trifluoride (BF₃), have a central atom with fewer than eight electrons in its outer shell.
- Expansion of the octet can occur for elements in Period 3 and below. These elements can use their d-orbitals to accommodate more than eight outer-shell electrons. Examples include phosphorus pentachloride (PCl₅) with ten outer electrons and sulfur hexafluoride (SF₆) with twelve.
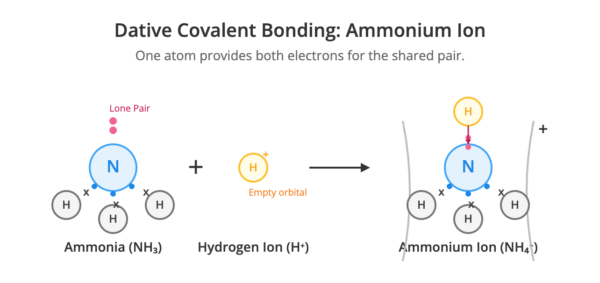
Dative (Co-ordinate) Covalent Bonding
A dative covalent bond is a type of covalent bond where one atom provides both of the shared electrons. This requires one atom to have a lone pair of electrons and another atom to have an empty orbital. An example is the formation of the ammonium ion (NH₄⁺), where the lone pair on the nitrogen atom in an ammonia molecule (NH₃) forms a bond with a hydrogen ion (H⁺).
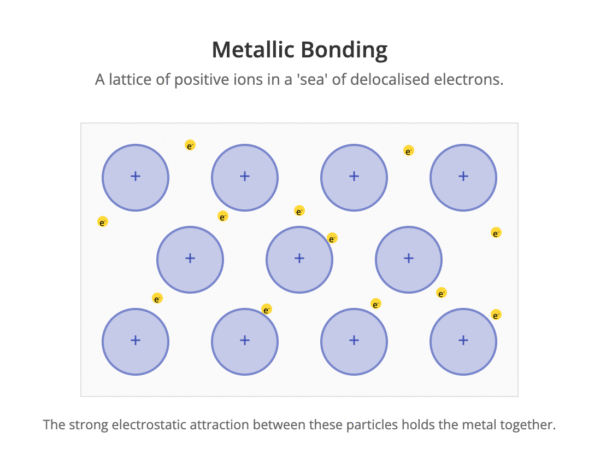
Metallic Bonding
Metallic bonding is the electrostatic attraction between a lattice of positive metal ions and a ‘sea’ of delocalised electrons. The outer shell electrons from the metal atoms are no longer associated with any single atom and are free to move throughout the entire metal structure. This strong, non-directional bonding accounts for the high melting points of most metals and their ability to conduct electricity and heat.