Quantum Subshells
The principal quantum shells (energy levels) in an atom, except for the very first one, are split into subshells. These are identified by the letters s, p, and d.
Each principal quantum shell contains a specific number of subshells, and within any given shell, the energy of the electrons increases in the order s < p < d.
- First principal shell (n=1): Contains one s subshell, holding a maximum of 2 electrons.
- Second principal shell (n=2): Contains an s subshell (2 electrons) and a p subshell (up to 6 electrons), for a total of 8 electrons.
- Third principal shell (n=3): Contains an s subshell (2 electrons), a p subshell (6 electrons), and a d subshell (up to 10 electrons), for a total of 18 electrons.
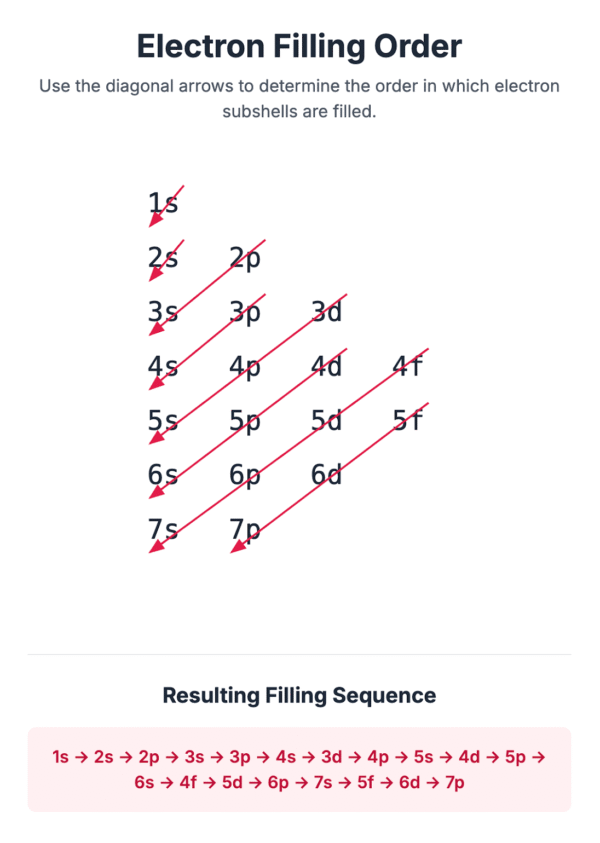
It is important to note that the energy levels of subshells start to overlap from the third principal shell onwards.
For instance, the 4s subshell is at a lower energy level than the 3d subshell, and so it is filled with electrons first.
Atomic Orbitals
Each subshell is made up of one or more atomic orbitals.
An atomic orbital is a region of space around the nucleus where there is a high probability of finding one or two electrons.
Each atomic orbital can hold a maximum of two electrons, which must have opposite spins. The number of atomic orbitals in each subshell is as follows:
- s subshell: Contains one s orbital.
- p subshell: Contains three p orbitals.
- d subshell: Contains five d orbitals.
Shapes of Orbitals
Atomic orbitals have distinct three-dimensional shapes.
- s orbitals are spherical. The 1s orbital is smaller than the 2s orbital, but both have the same spherical shape.
- p orbitals are shaped like an hourglass or dumbbell, with two “lobes”. The three p orbitals within a subshell (px, py, and pz) are arranged at right angles to each other along the x, y, and z axes. All three p orbitals in the same subshell have the same energy.
- d orbitals are shaped like a cloverleaf.
Filling Subshells and Orbitals
To achieve the most stable electronic configuration, electrons fill the subshells in order of increasing energy. The lowest energy subshell, 1s, is filled first. The order of filling follows the pattern shown in the energy level diagram, which accounts for the overlap between shells (e.g., 4s is filled before 3d).
Exam-Style Questions
- a) State the maximum number of electrons that can occupy a p subshell.
b) How many orbitals are there in a d subshell?
c) Describe the shape of an s orbital. - An atom of vanadium (V) has an atomic number of 23.
a) Write the full electronic configuration for a vanadium atom.
b) Identify the subshell that contains the highest energy electrons in a vanadium atom. - Explain why the 4s subshell is filled with electrons before the 3d subshell.
Answers
- a) A p subshell can hold a maximum of 6 electrons.
b) There are five orbitals in a d subshell.
c) An s orbital has a spherical shape. - a) The electronic configuration for vanadium (23 electrons) is 1s22s22p63s23p64s23d3.
b) The 3d subshell contains the highest energy electrons. Although the 4s subshell is filled first, the 3d subshell is at a higher energy level. - The energy levels of the principal quantum shells overlap. The 4s subshell is at a lower energy level than the 3d subshell. Electrons always fill the available subshell with the lowest energy first to achieve the most stable electronic configuration.
