Defining Atomic Mass
To compare the masses of different atoms, chemists use a standard scale. All atomic masses are measured relative to a standard atom, which is an isotope of carbon, carbon-12.
Unified Atomic Mass Unit (u)
The unified atomic mass unit is the standard unit for atomic masses. It is defined as exactly one-twelfth of the mass of a single, unbound neutral atom of carbon-12 in its ground state.
- 1 u is approximately equal to 1.66 x 10⁻²⁷ kg.
Relative Isotopic Mass
This refers to the mass of an atom of a specific isotope of an element, measured on the unified atomic mass unit scale. For example, the relative isotopic mass of chlorine-37 is approximately 37.
Relative Atomic Mass (Aᵣ)
Most elements exist naturally as a mixture of isotopes. The relative atomic mass is the weighted average mass of the atoms of an element compared to one-twelfth the mass of a carbon-12 atom.
- The formula is:
Aᵣ = (weighted average mass of atoms of an element) / (1/12 × mass of one carbon-12 atom) - Because Aᵣ is a ratio of masses, it has no units.
- The weighted average accounts for the different masses and the relative abundances of the isotopes. This is why the relative atomic mass of chlorine is 35.5, not a whole number.
Relative Molecular and Formula Mass (Mᵣ)
For molecules and ionic compounds, we use relative molecular mass or relative formula mass.
- It is calculated by summing the relative atomic masses of all the atoms present in the chemical formula.
- For example, the Mᵣ of methane (CH₄) is calculated as:
(1 × Aᵣ of C) + (4 × Aᵣ of H) = (1 × 12.0) + (4 × 1.0) = 16.0
Determining Relative Atomic Mass
The most accurate way to determine relative atomic masses is by using a mass spectrometer.
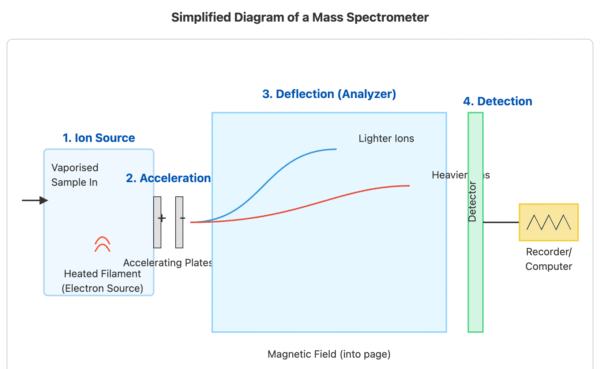
Mass Spectrometry
A mass spectrometer is an instrument that measures the mass and relative abundance of different isotopes within a sample of an element.
The process involves vaporising the sample, ionising the atoms, accelerating them, deflecting them with a magnetic field, and then detecting them. Heavier isotopes are deflected less than lighter ones.
The Mass Spectrum
The output from a mass spectrometer is a mass spectrum. This is a graph that plots the mass-to-charge (m/e) ratio against the relative abundance of the ions.
- For singly charged positive ions, the m/e ratio is equivalent to the mass number of the isotope.
- The height of each peak indicates the relative abundance of that isotope, often as a percentage.
Calculating Relative Atomic Mass from a Mass Spectrum
To calculate the relative atomic mass of an element from its mass spectrum, you follow a three-step process:
- Multiply the mass-to-charge ratio of each isotope by its percentage abundance.
- Add these values together.
- Divide the total by 100.
Example Calculation (for Neon)
A sample of neon has three isotopes with the following abundances:
- ²⁰Ne: 90.9%
- ²¹Ne: 0.3%
- ²²Ne: 8.8%
Aᵣ of Neon = [(20 × 90.9) + (21 × 0.3) + (22 × 8.8)] / 100 = 20.2
This calculation demonstrates how the presence and abundance of different isotopes result in a non-integer value for the relative atomic mass of an element.
