Stoichiometry is the study of the quantitative relationships between the amounts of reactants and products in a chemical reaction. The concept of the mole is central to all stoichiometric calculations.
Relative Masses of Atoms and Molecules
- Relative Atomic Mass (Ar): The weighted average mass of an atom of an element, relative to one-twelfth of the mass of a carbon-12 atom. It is a ratio and has no units.
- Relative Molecular Mass (Mr): The sum of the relative atomic masses of all the atoms present in one molecule of a compound. For ionic compounds, this is referred to as the Relative Formula Mass.
The Mole and Avogadro’s Constant
A mole is the standard unit for the amount of a substance. One mole of any substance contains the same number of specified particles (atoms, molecules, ions, or electrons). This number is known as the Avogadro constant (L), which has a value of 6.02 × 10²³ mol⁻¹.
The mass of one mole of a substance is its molar mass (M), measured in g mol⁻¹. The molar mass in grams is numerically equal to the substance’s relative formula mass (Mr).
The relationship between mass, moles, and molar mass is fundamental to the concept of the mole.
Amount of substance (mol) = Mass (g) / Molar Mass (g mol⁻¹)
Worked Examples
- How many moles are in 4.0 g of sodium hydroxide (NaOH)?
(Ar values: Na = 23.0, O = 16.0, H = 1.0)
- Molar Mass of NaOH = 23.0 + 16.0 + 1.0 = 40.0 g mol⁻¹
- Amount = Mass / Molar Mass = 4.0 g / 40.0 g mol⁻¹ = 0.10 mol
- What is the mass of 0.25 mol of calcium carbonate (CaCO₃)?
(Ar values: Ca = 40.1, C = 12.0, O = 16.0)
- Molar Mass of CaCO₃ = 40.1 + 12.0 + (3 × 16.0) = 100.1 g mol⁻¹
- Mass = Amount × Molar Mass = 0.25 mol × 100.1 g mol⁻¹ = 25.0 g (to 3 s.f.)
- How many atoms are there in 6.0 g of carbon (C)?
(Ar value: C = 12.0)
- Amount = Mass / Molar Mass = 6.0 g / 12.0 g mol⁻¹ = 0.50 mol
- Number of atoms = Amount × Avogadro constant = 0.50 mol × (6.02 × 10²³ mol⁻¹) = 3.01 × 10²³ atoms
Chemical Formulae
- Empirical Formula: The simplest whole-number ratio of atoms of each element in a compound.
- Molecular Formula: The actual number of atoms of each element in one molecule of a compound. It is always a whole-number multiple of the empirical formula.
To determine the empirical formula from composition data (by mass or percentage):
- List the mass or percentage of each element.
- Divide each value by the element’s relative atomic mass (Ar) to find the molar ratio.
- Divide each number in the ratio by the smallest number to get the simplest ratio.
- If necessary, multiply to get a whole-number ratio.
The molecular formula can be found by dividing the relative molecular mass by the empirical formula mass to find the multiplier.
Reacting Mass Calculations
A balanced chemical equation provides the molar ratio, or stoichiometry, of reactants and products. This ratio is used to calculate the masses of substances involved in a reaction.
In many reactions, one reactant is completely used up before the others. This is the limiting reagent, and it determines the maximum amount of product that can be formed. Reactants that are not completely used up are in excess.
The percentage yield compares the actual amount of product obtained experimentally to the theoretical maximum amount predicted by stoichiometry.
Percentage Yield = (Actual Yield / Theoretical Yield) × 100
Worked Examples
- In an experiment, 1.27 g of copper reacts with excess sulfur to produce 1.45 g of copper(I) sulfide. What is the percentage yield?
Equation: 2Cu(s) + S(s) → Cu₂S(s)
(Ar values: Cu = 63.5, S = 32.1)
- Amount of Cu = 1.27 g / 63.5 g mol⁻¹ = 0.0200 mol
- From stoichiometry, 2 mol Cu produces 1 mol Cu₂S. So, 0.0200 mol Cu produces 0.0100 mol Cu₂S.
- Theoretical Yield of Cu₂S = 0.0100 mol × (2 × 63.5 + 32.1) g mol⁻¹ = 1.59 g
- Percentage Yield = (1.45 g / 1.59 g) × 100 = 91.2%
- A student reacts 5.60 g of iron with 5.12 g of sulfur to produce iron(II) sulfide (FeS). What is the theoretical yield and which is the limiting reagent?
Equation: Fe(s) + S(s) → FeS(s)
(Ar values: Fe = 55.8, S = 32.1)
- Amount of Fe = 5.60 g / 55.8 g mol⁻¹ = 0.100 mol
- Amount of S = 5.12 g / 32.1 g mol⁻¹ = 0.160 mol
- The molar ratio is 1:1, so iron is the limiting reagent as there is less of it.
- Max moles of FeS produced = 0.100 mol
- Theoretical Yield = 0.100 mol × (55.8 + 32.1) g mol⁻¹ = 8.79 g
- The synthesis of aspirin has a percentage yield of 75.0%. If a chemist starts with 13.8 g of salicylic acid, what is the actual yield of aspirin?
(Salicylic acid Mr = 138.0, Aspirin Mr = 180.0)
Equation: C₇H₆O₃ + C₄H₆O₃ → C₉H₈O₄ + C₂H₄O₂
- Amount of salicylic acid = 13.8 g / 138.0 g mol⁻¹ = 0.100 mol
- From stoichiometry (1:1), theoretical moles of aspirin = 0.100 mol
- Theoretical Yield = 0.100 mol × 180.0 g mol⁻¹ = 18.0 g
- Actual Yield = (Percentage Yield / 100) × Theoretical Yield = (75.0 / 100) × 18.0 g = 13.5 g
Solutions and Concentration
The concentration of a solution is the amount of solute dissolved in a given volume of solution. It is typically expressed in moles per cubic decimetre (mol dm⁻³).
Concentration (mol dm⁻³) = Amount of substance (mol) / Volume (dm³)
Note that 1 dm³ = 1000 cm³. This formula is frequently used in titration calculations to determine the unknown concentration of a solution.
Worked Examples
- Calculate the concentration of a solution made by dissolving 5.85 g of NaCl in 200 cm³ of water.
(Mr of NaCl = 58.5)
- Amount of NaCl = 5.85 g / 58.5 g mol⁻¹ = 0.100 mol
- Volume = 200 cm³ = 0.200 dm³
- Concentration = 0.100 mol / 0.200 dm³ = 0.500 mol dm⁻³
- In a titration, 25.0 cm³ of NaOH solution required 22.5 cm³ of 0.100 mol dm⁻³ HCl for neutralisation. What is the concentration of the NaOH?
- Amount of HCl = 0.100 mol dm⁻³ × (22.5 / 1000) dm³ = 0.00225 mol
- Equation: NaOH + HCl → NaCl + H₂O. The ratio is 1:1.
- Amount of NaOH = 0.00225 mol
- Concentration of NaOH = 0.00225 mol / (25.0 / 1000) dm³ = 0.0900 mol dm⁻³
- What mass of potassium hydroxide (KOH) is needed to prepare 250 cm³ of a 0.200 mol dm⁻³ solution?
(Mr of KOH = 56.1)
- Amount of KOH needed = 0.200 mol dm⁻³ × (250 / 1000) dm³ = 0.0500 mol
- Mass of KOH = 0.0500 mol × 56.1 g mol⁻¹ = 2.81 g
Gas Volumes in Calculations
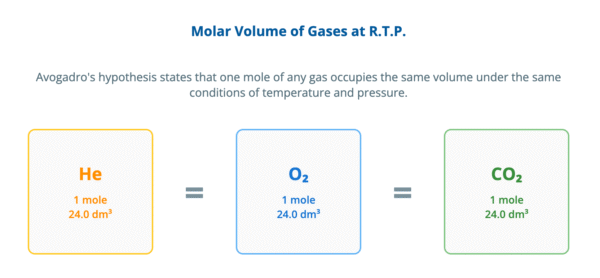
Avogadro’s hypothesis states that equal volumes of all gases, at the same temperature and pressure, contain the same number of molecules.
From this, it follows that one mole of any gas occupies a fixed volume under specific conditions. At room temperature and pressure (r.t.p.), this is the molar gas volume, which is 24.0 dm³ mol⁻¹.
This relationship allows for direct conversion between the amount of a gas in moles and its volume.
Volume of gas (dm³) = Amount of substance (mol) × 24.0
The stoichiometry of reactions involving gases can also be determined by comparing the volumes of the reacting gases, as the ratio of volumes is equal to the ratio of moles.
Worked Examples
- What is the volume of 0.50 mol of nitrogen gas (N₂) at r.t.p.?
- Volume = Amount × Molar Volume = 0.50 mol × 24.0 dm³ mol⁻¹ = 12.0 dm³
- What is the mass of 480 cm³ of carbon dioxide (CO₂) gas at r.t.p.?
(Mr of CO₂ = 44.0)
- Volume = 480 cm³ = 0.480 dm³
- Amount = Volume / Molar Volume = 0.480 dm³ / 24.0 dm³ mol⁻¹ = 0.0200 mol
- Mass = 0.0200 mol × 44.0 g mol⁻¹ = 0.880 g
- Calculate the volume of CO₂ produced at r.t.p. when 6.0 g of magnesium carbonate decomposes on heating.
Equation: MgCO₃(s) → MgO(s) + CO₂(g)
(Mr of MgCO₃ = 84.3)
- Amount of MgCO₃ = 6.0 g / 84.3 g mol⁻¹ = 0.0712 mol
- From stoichiometry (1:1), Amount of CO₂ produced = 0.0712 mol
- Volume of CO₂ = 0.0712 mol × 24.0 dm³ mol⁻¹ = 1.71 dm³
Exam-Style Questions
1. A hydrocarbon contains 85.7% carbon by mass. Its relative molecular mass is 70.0.
(a) Calculate the empirical formula of the hydrocarbon.
(b) Calculate the molecular formula of the hydrocarbon.
(Ar values: C = 12.0, H = 1.0)
2. 25.0 cm³ of a 0.150 mol dm⁻³ solution of sodium carbonate was titrated against hydrochloric acid. The volume of acid required for complete neutralisation was 28.5 cm³.
The equation for the reaction is:
Na₂CO₃(aq) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g)
(a) Calculate the number of moles of sodium carbonate in the 25.0 cm³ sample.
(b) Calculate the number of moles of hydrochloric acid that reacted.
(c) Calculate the concentration of the hydrochloric acid in mol dm⁻³.
3. 1.12 g of iron powder is added to 50.0 cm³ of 0.500 mol dm⁻³ copper(II) sulfate solution.
The equation for the reaction is:
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
(a) Determine which reactant is the limiting reagent. Show your working.
(b) Calculate the mass of copper that is formed.
(c) The reaction is exothermic. If the initial temperature was 21.0 °C and the final temperature was 32.5 °C, calculate the heat energy produced, assuming the specific heat capacity of the solution is 4.18 J g⁻¹ °C⁻¹ and its density is 1.00 g cm⁻³.
(Ar values: Fe = 55.8, Cu = 63.5, S = 32.1, O = 16.0)
