Defining Isotopes
Isotopes are atoms of the same element that possess the same number of protons but a different number of neutrons. This means they share the same atomic (proton) number (Z) but have different mass (nucleon) numbers (A).
Because isotopes of an element have the same number of protons, they also have the same number of electrons in a neutral atom. The identity of an element is determined solely by its atomic number.
Isotopes are represented using the following notation:
ᴬzX
Where:
- X is the chemical symbol of the element.
- A is the mass (nucleon) number (total number of protons and neutrons).
- Z is the atomic (proton) number (total number of protons).
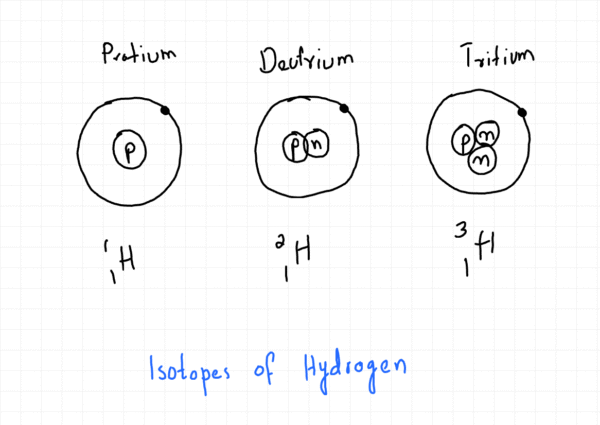
For example, the isotopes of hydrogen are hydrogen-1 (¹₁H), hydrogen-2 (²₁H), and hydrogen-3 (³₁H).
Properties of Isotopes
Chemical Properties
Isotopes of the same element exhibit identical chemical properties. This is because chemical reactions involve the electrons of an atom, particularly the valence electrons.
Since all isotopes of an element have the same number of protons, they have the same number of electrons and the same electronic configuration. Therefore, they react in the same way.
Physical Properties
While their chemical properties are the same, isotopes of an element have different physical properties. These differences are due to their different masses.
- Mass: Isotopes have different masses because they contain a different number of neutrons. For instance, hydrogen-2 is twice as heavy as hydrogen-1.
- Density: Due to the difference in mass, isotopes and their compounds can have slightly different densities.
These variations in physical properties are generally minor but can be significant in certain applications, such as in radioactive dating or in the production of heavy water.
