Ionization energy is a fundamental concept that provides strong evidence for the arrangement of electrons in shells and sub-shells within an atom. It measures the energy required to remove electrons from gaseous atoms or ions.
First Ionization Energy
The first ionization energy (IE₁) is defined as the energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions.
The process can be represented by the general equation:
X(g)→X+(g) + e−
For example, for calcium:
Ca(g)→Ca+(g)+e− IE1=+590 kJ mol⁻¹
It is an endothermic process, so ionization energy values are always positive.
Successive Ionization Energies
After the first electron is removed, it is possible to remove further electrons from the resulting positive ion. This gives rise to successive ionization energies.
- Second Ionization Energy (IE₂): The energy needed to remove one electron from each ion in one mole of gaseous 1+ ions.
- Third Ionization Energy (IE₃): The energy needed to remove one electron from each ion in one mole of gaseous 2+ ions.
Successive ionization energies for an element always increase. This is because as electrons are removed, the remaining electrons are pulled closer by the unchanged nuclear charge. The effective positive charge of the ion increases, making it more difficult to remove the next electron.
A key feature of successive ionization energy data is the presence of large jumps in value. These jumps occur when an electron is removed from a principal quantum shell that is closer to the nucleus. This provides direct evidence for the existence of electron shells.
By analyzing the pattern of successive ionization energies, we can determine the number of valence electrons and therefore deduce the group an element belongs to in the Periodic Table. For example, a large jump between the second and third ionization energies indicates that the element has two valence electrons and is in Group 2.
Factors Influencing Ionization Energy
Four main factors affect the magnitude of ionization energy:
- Nuclear Charge: The greater the number of protons in the nucleus, the stronger the electrostatic attraction for the outer electrons. A higher nuclear charge leads to a higher ionization energy.
- Atomic Radius: The greater the distance between the nucleus and the outer electrons, the weaker the electrostatic attraction. A larger atomic radius leads to a lower ionization energy.
- Shielding: Inner shells of electrons repel the outer-shell electrons, shielding them from the full attraction of the nucleus. Increased shielding significantly lowers the ionization energy.
- Spin-pair Repulsion: Electrons in the same orbital repel each other. This repulsion makes it slightly easier to remove an electron from a shared orbital compared to a singly occupied one. This effect slightly lowers the ionization energy.
Trends in First Ionization Energy
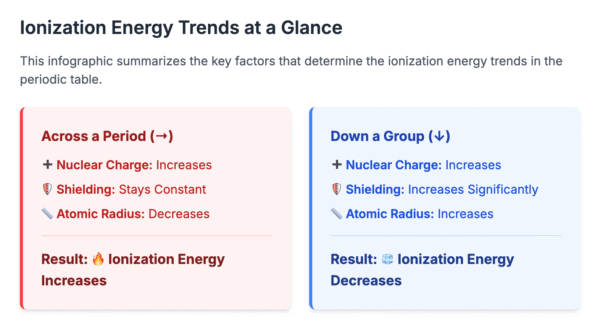
Across a Period
There is a general increase in the first ionization energy as you move from left to right across a period.
Reason: The nuclear charge increases, and electrons are added to the same principal shell. This pulls the electrons closer to the nucleus (decreasing atomic radius) and increases the electrostatic attraction, making it harder to remove an electron. Shielding by inner electrons remains relatively constant.
There are two main anomalies to this trend:
- Dip between Group 2 and Group 13: For example, the IE₁ of aluminium is lower than that of magnesium. This is because the outermost electron in aluminium is in a 3p sub-shell, which is at a slightly higher energy level and further from the nucleus than the 3s sub-shell of magnesium. The 3p electron also experiences extra shielding from the 3s electrons.
- Dip between Group 15 and Group 16: For example, the IE₁ of sulfur is lower than that of phosphorus. This is due to spin-pair repulsion. In phosphorus, the three 3p electrons are in separate orbitals. In sulfur, the fourth 3p electron must pair up in one of the orbitals. The repulsion between these paired electrons makes it slightly easier to remove one of them.
Down a Group
The first ionization energy decreases as you go down a group.
Reason: Although the nuclear charge increases, this effect is outweighed by the increase in atomic radius and the significant increase in shielding from the additional inner electron shells. The outermost electron is further from the nucleus and feels a weaker attraction, making it easier to remove.
Exam-Style Questions
1. Define the term ‘first ionization energy’ and explain the general trend in first ionization energies down Group 2.
Answer:
The first ionization energy is the energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous 1+ ions. Down Group 2, the first ionization energy decreases. This is because, although the nuclear charge increases, the number of inner electron shells also increases, leading to greater shielding. The atomic radius increases, so the outermost electron is further from the nucleus. These factors reduce the electrostatic attraction between the nucleus and the outer electron, making it easier to remove.
2. The first five successive ionization energies of an element, Y, are 736, 1450, 7740, 10500, and 13600 kJ mol⁻¹. In which group of the Periodic Table is element Y found? Explain your reasoning.
Answer:
Element Y is in Group 2. There is a large increase in ionization energy between the second (1450 kJ mol⁻¹) and third (7740 kJ mol⁻¹) values. This indicates that the third electron is being removed from a principal quantum shell closer to the nucleus, which requires significantly more energy. This means the element has two valence electrons in its outer shell, placing it in Group 2.
3. Explain why the first ionization energy of sulfur is lower than that of phosphorus.
Answer:
The electronic configuration of phosphorus is [Ne] 3s² 3pₓ¹ 3pᵧ¹ 3pz¹. The electronic configuration of sulfur is [Ne] 3s² 3pₓ² 3pᵧ¹ 3pz¹. To ionize sulfur, the electron is removed from the paired set of electrons in the 3pₓ orbital. The repulsion between these two electrons in the same orbital (spin-pair repulsion) means that less energy is required to remove one of them compared to removing an electron from a half-filled orbital in phosphorus.
