Ionic Bonding
Ionic bonding is the electrostatic force of attraction between oppositely charged ions within a giant ionic lattice structure. This type of bond typically forms between a metallic element and a non-metallic element.
Formation of Ions
The formation of ionic and covalent compounds is driven by the tendency of atoms to achieve a more stable electron configuration, usually that of the nearest noble gas.
In ionic bonding, this is achieved through the transfer of one or more electrons from the outer shell of a metal atom to the outer shell of a non-metal atom.
- The metal atom loses electrons to form a positively charged ion, known as a cation.
- The non-metal atom gains these electrons to form a negatively charged ion, known as an anion.
For example, in the formation of sodium chloride, a sodium atom transfers its single outer electron to a chlorine atom. The resulting Na⁺ ion has the electron configuration of neon, and the Cl⁻ ion has the electron configuration of argon.
Dot-and-Cross Diagrams
Dot-and-cross diagrams are used to represent the arrangement of outer shell electrons in ionic compounds. For an ionic compound, the diagram should show:
- The outer electron shell of each ion.
- Square brackets around each ion to indicate that the charge is spread over the ion.
- The charge on each ion written as a superscript outside the top right of the bracket.
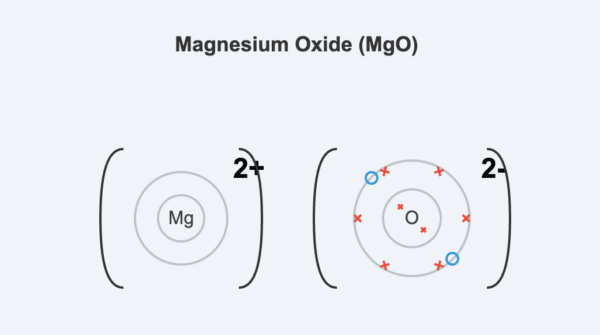
Example: Magnesium Oxide (MgO)
A magnesium atom transfers its two outer electrons to an oxygen atom. The Mg²⁺ ion and the O²⁻ ion are formed, both achieving a stable noble gas configuration.
Example: Calcium Fluoride (CaF₂)
A calcium atom transfers one outer electron to each of two fluorine atoms. This forms one Ca²⁺ ion and two F⁻ ions.
Covalent Bonding
Covalent bonding involves the sharing of one or more pairs of electrons between atoms, typically non-metals. The shared pair of electrons is attracted to the positive nuclei of both atoms, holding them together.
Single, Double, and Triple Bonds
- A single covalent bond consists of one shared pair of electrons (e.g., in H₂, CH₄).
- A double covalent bond consists of two shared pairs of electrons (e.g., in O₂, CO₂).
- A triple covalent bond consists of three shared pairs of electrons (e.g., in N₂).
Double and triple bonds are generally shorter and stronger than single bonds between the same two elements.
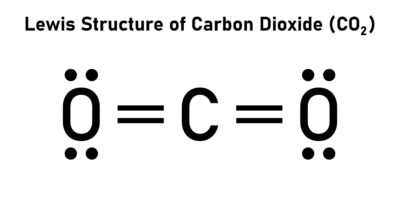
Lone Pairs and Bond Pairs
In a molecule, the pairs of outer shell electrons that are not involved in bonding are called lone pairs. The shared pairs of electrons that form the covalent bonds are called bond pairs. For example, in a molecule of ammonia (NH₃), the nitrogen atom has three bond pairs and one lone pair.
Exceptions to the Octet Rule
While many atoms in covalent molecules have eight electrons in their outer shell, there are exceptions.
- Electron Deficiency: Some atoms, like boron in boron trifluoride (BF₃), can be stable with fewer than eight outer electrons. Boron has only six electrons in its outer shell in this compound.
- Expansion of the Octet: Elements in Period 3 and below can accommodate more than eight electrons in their outer shell by utilising their d-orbitals. Examples include phosphorus in phosphorus pentachloride (PCl₅) with ten outer electrons, and sulfur in sulfur hexafluoride (SF₆) with twelve outer electrons.
Comparison of Ionic and Covalent Compounds
| Property | Ionic Compounds | Simple Covalent Compounds |
| Structure | Giant ionic lattice. | Simple discrete molecules. |
| Bonding | Strong electrostatic forces of attraction between oppositely charged ions. | Strong covalent bonds within molecules; weak intermolecular forces between molecules. |
| Melting & Boiling Points | High, as a large amount of energy is needed to overcome the strong ionic bonds. | Low, as only weak intermolecular forces need to be overcome. |
| Electrical Conductivity | Conduct electricity only when molten or dissolved in water, as ions are free to move. | Do not conduct electricity, as there are no free-moving charged particles. |
| Solubility in Water | Often soluble, as polar water molecules can surround and stabilise the ions. | Generally insoluble unless they can form hydrogen bonds with water. |
