Formal Charge Calculator
Determine the formal charge of an atom in a molecule.
Formula
FC = V – N – (B/2)
Calculated Formal Charge (FC)
—
Formal Charge in Chemistry
In chemistry, the formal charge is a theoretical charge assigned to an individual atom within a molecule or ion. It’s a tool used to track electron distribution in Lewis structures, helping chemists determine the most plausible or stable arrangement of atoms and electrons when multiple possibilities exist. The concept assumes that electrons in a covalent bond are shared perfectly equally between atoms, which is a useful simplification but not always a reflection of physical reality.
The primary goal of calculating formal charge is to identify the Lewis structure where the atoms have formal charges as close to zero as possible.
The Formal Charge Formula
The calculation is straightforward and relies on a simple formula that compares the electrons an atom “owns” in a molecule to the electrons it has as a neutral, free atom.
Breaking Down the Formula
The formula is expressed as:
FC = V – N – (B/2)
Let’s look at what each variable represents:
- FC (Formal Charge): The final calculated charge for the atom in question.
- V (Valence Electrons): The number of valence electrons the atom has in its neutral state, as found on the periodic table.
- N (Non-bonding Electrons): The number of valence electrons on the atom that are not involved in bonding (i.e., the electrons in lone pairs).
- B (Bonding Electrons): The total number of electrons that the atom shares in covalent bonds with other atoms.
How to Calculate Formal Charge: Step-by-Step Examples
The best way to understand the process is to walk through a few examples. A correct Lewis structure is essential before you begin.
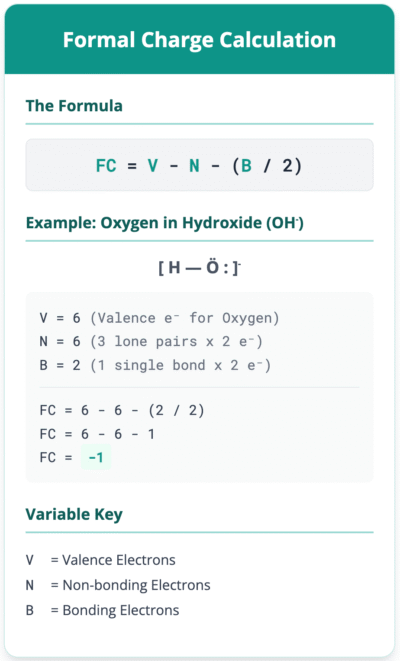
Example 1: The Oxygen Atom in a Hydroxide Ion (OH⁻)
Let’s find the formal charge of the oxygen atom in the hydroxide ion.
- Valence Electrons (V): A neutral oxygen atom is in Group 16, so it has 6 valence electrons.
- Non-bonding Electrons (N): In the Lewis structure, oxygen has three lone pairs. Each pair has 2 electrons, so there are 3 * 2 = 6 non-bonding electrons.
- Bonding Electrons (B): Oxygen forms one single covalent bond with hydrogen, which consists of 2 shared electrons.
- Apply the Formula:
- FC = V – N – (B/2)
- FC = 6 – 6 – (2/2)
- FC = 6 – 6 – 1
- FC = -1
The formal charge on the oxygen atom in the hydroxide ion is -1.
Example 2: The Nitrogen Atom in an Ammonium Ion (NH₄⁺)
Now, let’s determine the formal charge of the central nitrogen atom in the ammonium ion.
- Valence Electrons (V): A neutral nitrogen atom is in Group 15, so it has 5 valence electrons.
- Non-bonding Electrons (N): In this structure, nitrogen has no lone pairs, so it has 0 non-bonding electrons.
- Bonding Electrons (B): Nitrogen forms four single covalent bonds with four hydrogen atoms. The total number of shared electrons is 4 * 2 = 8.
- Apply the Formula:
- FC = V – N – (B/2)
- FC = 5 – 0 – (8/2)
- FC = 5 – 0 – 4
- FC = +1
The formal charge on the nitrogen atom in the ammonium ion is +1.
Using Formal Charge to Predict the Best Molecular Structure
When you can draw more than one valid Lewis structure for a molecule (these are called resonance structures), formal charges help you decide which one is the most significant or stable contributor. The best structure generally follows these rules:
- The structure with formal charges on all atoms closest to zero is preferred.
- A structure with negative formal charges on the more electronegative atoms is more stable.
- Structures that place adjacent atoms with like charges (e.g., +1 next to +1) are highly unfavorable.
Frequently Asked Questions (FAQs)
What is the difference between formal charge and oxidation state?
Formal charge and oxidation state are both bookkeeping tools for electrons, but they operate on different assumptions. Formal charge assumes electrons in a bond are shared perfectly equally (a pure covalent model). Oxidation state, conversely, assumes the more electronegative atom in a bond gets all the electrons (a pure ionic model). The reality for most bonds is somewhere in between.
Can the formal charge of an atom be a fraction?
No. The number of valence, non-bonding, and bonding electrons are always integers. Since the number of bonding electrons (B) in any stable molecule is always an even number, dividing it by 2 always results in an integer. Therefore, the final formal charge will always be an integer (e.g., -2, -1, 0, +1, +2).
Is the sum of all formal charges equal to the overall charge?
Yes, always. For a neutral molecule, the sum of the formal charges of all its atoms must be zero. For a polyatomic ion, the sum must equal the ion’s charge. This serves as an excellent way to double-check your calculations.
Why is it important to draw the correct Lewis structure first?
The calculation of formal charge is entirely dependent on the number of bonding and non-bonding electrons shown in a specific Lewis structure. If the Lewis structure is drawn incorrectly (e.g., with the wrong number of bonds or lone pairs on an atom), the resulting formal charges will also be incorrect.
