Representing Electronic Configurations
The electronic configuration of an atom shows how electrons are arranged in their shells, sub-shells, and orbitals. We use a specific notation to represent this arrangement. For example, the electronic configuration of a hydrogen atom is written as 1s¹.
- The first number (1) indicates the principal quantum number or shell.
- The letter (s) refers to the sub-shell.
- The superscript number (¹) shows the number of electrons in that sub-shell.
For elements with many electrons, a shorthand notation is often used. The electronic configuration of the preceding noble gas is represented by its symbol in square brackets, followed by the configuration of the outer electrons.
For example, the full electronic configuration of potassium is 1s²2s²2p⁶3s²3p⁶4s¹, but it can be written in shorthand as [Ar] 4s¹.
Filling Orbitals and Sub-shells
To determine the electronic configuration of an atom, electrons are added to sub-shells in order of increasing energy. The lowest energy sub-shell, 1s, is filled first.
The general order for filling sub-shells is:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p…
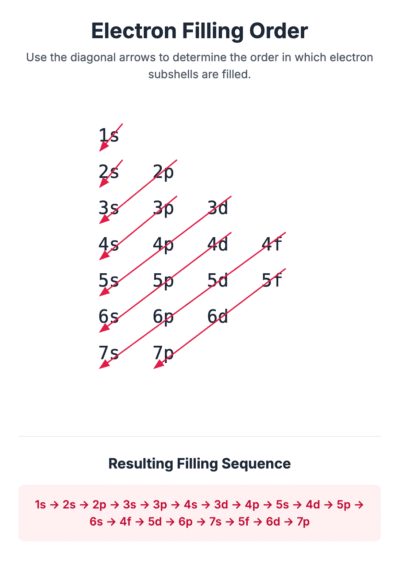
Notice that the 4s sub-shell has a lower energy level than the 3d sub-shell, so it is filled first.
When filling orbitals within the same sub-shell (e.g., the three p orbitals), remember these rules:
- Electrons occupy separate orbitals first before they start pairing up.
- When electrons are paired in an orbital, they must have opposite spins.
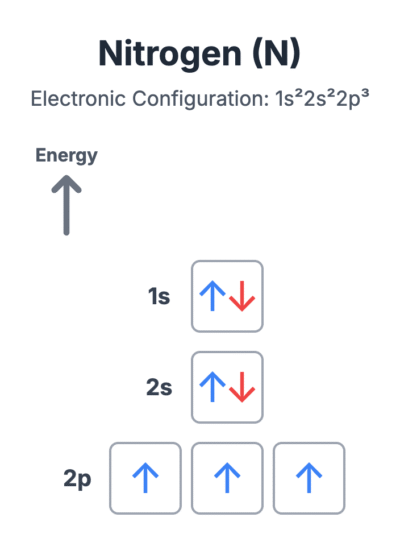
The ‘Electrons in Boxes’ Notation
Another way to represent electronic configurations is the ‘electrons in boxes’ notation. Each box represents an atomic orbital, and an arrow represents an electron. The direction of the arrow indicates the electron’s spin.
- An orbital with one electron has a single arrow: ⬆
- An orbital with two electrons has two arrows pointing in opposite directions: ⬆⬇
This notation clearly shows how electrons are distributed within the orbitals of a sub-shell. For example, the electronic configuration of a nitrogen atom (1s²2s²2p³) is shown with its 2p electrons in separate orbitals with the same spin.
Electronic Configurations of Ions
The electronic configurations of ions are determined by adding or removing electrons from the atom’s configuration.
- Negative ions (anions) are formed when an atom gains electrons. The added electrons fill the available orbitals in the lowest available energy level. For example, a sulfur atom ([Ne] 3s²3p⁴) gains two electrons to form a sulfide ion, S²⁻, with the configuration [Ne] 3s²3p⁶.
- Positive ions (cations) are formed when an atom loses electrons. Electrons are removed from the highest energy level first. For d-block elements, the 4s electrons are removed before the 3d electrons. For example, an iron atom ([Ar] 3d⁶4s²) loses its two 4s electrons to form an Fe²⁺ ion with the configuration [Ar] 3d⁶. To form an Fe³⁺ ion, it loses the two 4s electrons and one 3d electron, resulting in the configuration [Ar] 3d⁵.
Exceptions to the Rule
The electronic configurations of chromium (Cr) and copper (Cu) do not follow the expected pattern. This is because a half-filled (d⁵) or completely filled (d¹⁰) d sub-shell is more energetically stable.
- Chromium (Cr): The expected configuration is [Ar] 3d⁴4s². However, the actual configuration is [Ar] 3d⁵4s¹. One electron moves from the 4s sub-shell to the 3d sub-shell to achieve a more stable half-filled d sub-shell.
- Copper (Cu): The expected configuration is [Ar] 3d⁹4s². The actual configuration is [Ar] 3d¹⁰4s¹. An electron is promoted from the 4s sub-shell to achieve a more stable, completely filled d sub-shell.
