Dot-and-cross diagrams are a simple way to model the arrangement of outer shell electrons in atoms and ions. They are used to illustrate how electrons are involved in chemical bonding. In these diagrams, electrons from one atom are shown as dots (•) and electrons from another atom are shown as crosses (x). This helps to track the origin of the electrons in a bond, although it is important to remember that all electrons are identical.
These diagrams typically only show the valence (outermost) electron shell.
Representing Ionic Bonding
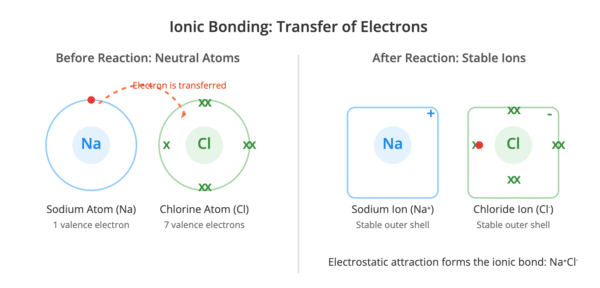
In ionic bonding, dot-and-cross diagrams show the transfer of electrons from a metal atom to a non-metal atom.
The key features when drawing dot-and-cross diagrams for ionic compounds are:
- The metal atom loses its outer shell electrons, and these are transferred to the non-metal atom.
- The resulting ions are drawn separately, often with the metal ion shown with an empty outer shell.
- Square brackets are placed around each ion.
- The charge on each ion is written at the top right-hand corner, outside the brackets.
For example, in sodium chloride, the sodium atom transfers its single outer electron to the chlorine atom to form Na⁺ and Cl⁻ ions, both with stable, full outer shells.
For a compound like magnesium oxide, the magnesium atom transfers two electrons to the oxygen atom, resulting in Mg²⁺ and O²⁻ ions.
Representing Covalent Bonding
For covalent molecules, dot-and-cross diagrams show how electrons are shared between non-metal atoms to form covalent bonds. The area where the electron shells overlap represents the shared pair of electrons, known as a bond pair. Pairs of outer electrons not involved in bonding are called lone pairs.
Single Covalent Bonds
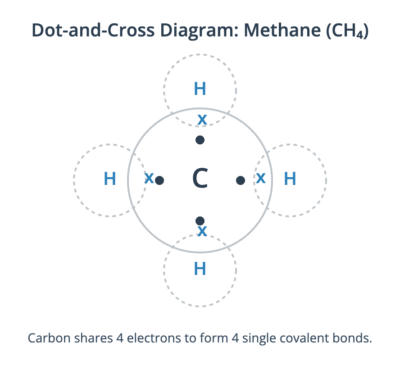
A single covalent bond is formed when two atoms share one pair of electrons. Many common molecules contain single bonds, such as methane (CH₄), where a central carbon atom shares a pair of electrons with each of four hydrogen atoms.
Multiple Covalent Bonds
Atoms can share more than one pair of electrons to achieve a stable octet.
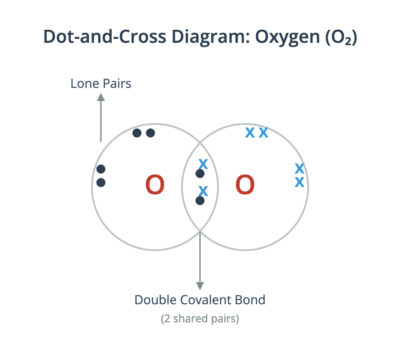
- A double covalent bond is the sharing of two pairs of electrons, as seen in an oxygen molecule (O₂).
- A triple covalent bond is the sharing of three pairs of electrons, as seen in a nitrogen molecule (N₂).
Molecules with Exceptions to the Octet Rule
Dot-and-cross diagrams can also represent molecules that do not follow the octet rule.
- Some central atoms can be electron-deficient, having fewer than eight electrons in their outer shell. Boron trifluoride (BF₃) is a common example, where boron only has six outer electrons.
- Elements in Period 3 and below can expand their octet by using available d-orbitals. Sulfur hexafluoride (SF₆) is an example where the central sulfur atom has twelve electrons in its outer shell.
