Dot-and-Cross Diagrams Notes | CIE | A-Level Chemistry
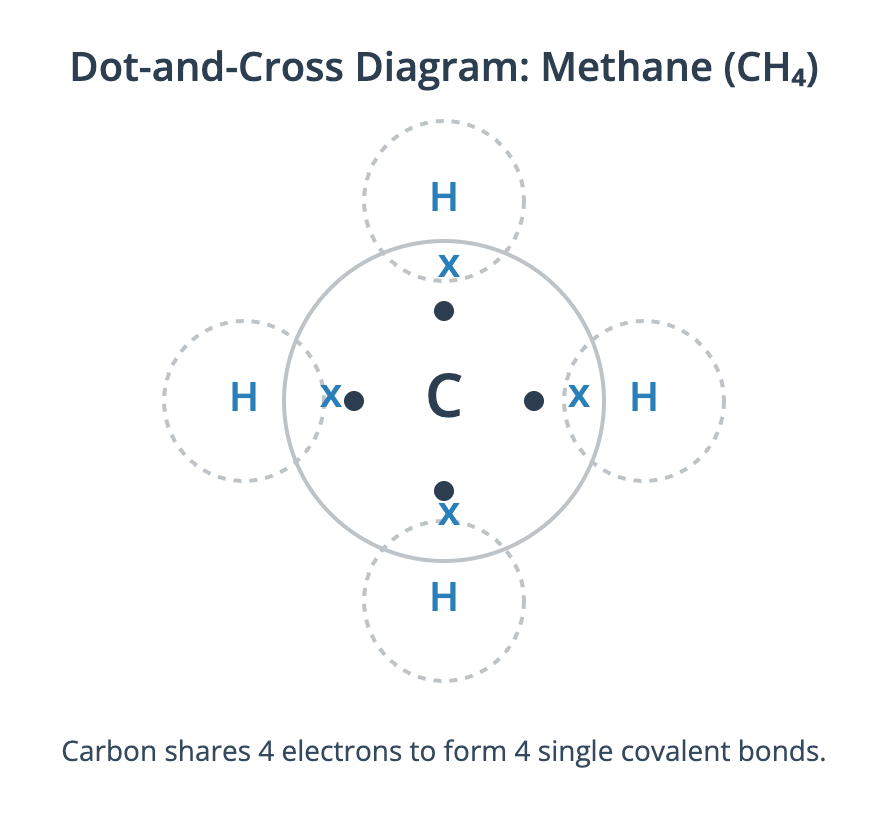
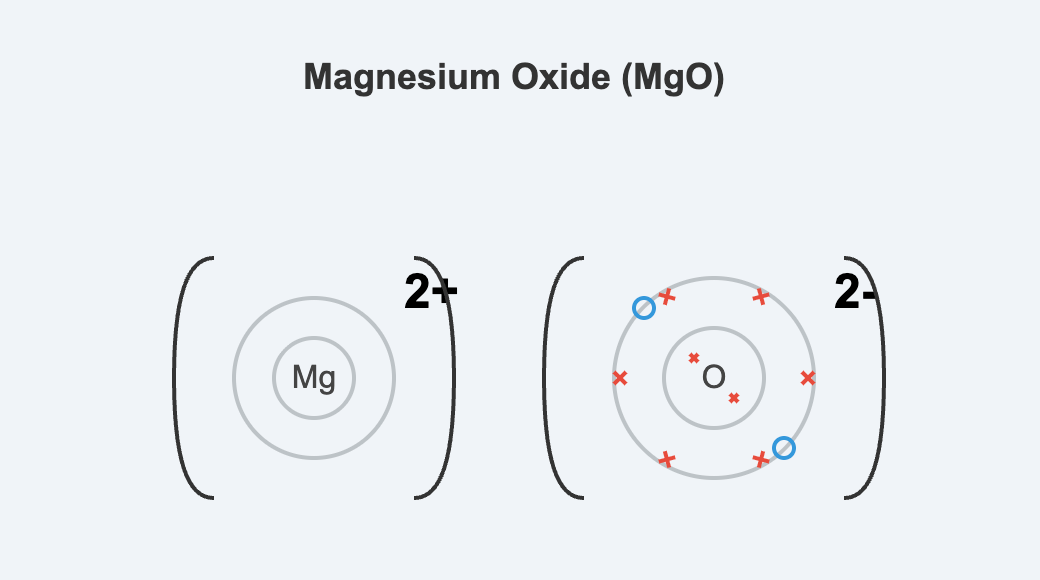
Dot-and-cross diagrams are a simple way to model the arrangement of outer shell electrons in atoms and ions. They are used to illustrate how electrons are involved in chemical bonding. In these diagrams, electrons from one atom are shown as dots (•) and electrons from another atom are shown as crosses (x). This helps to track the origin of the electrons in a bond, although it is important to remember that all electrons are identical. These diagrams typically only show the valence (outermost) electron shell. Representing Ionic Bonding In ionic bonding, dot-and-cross diagrams show the transfer of electrons from a
Types of Bonding Revision Notes | CIE | A-Level Chemistry
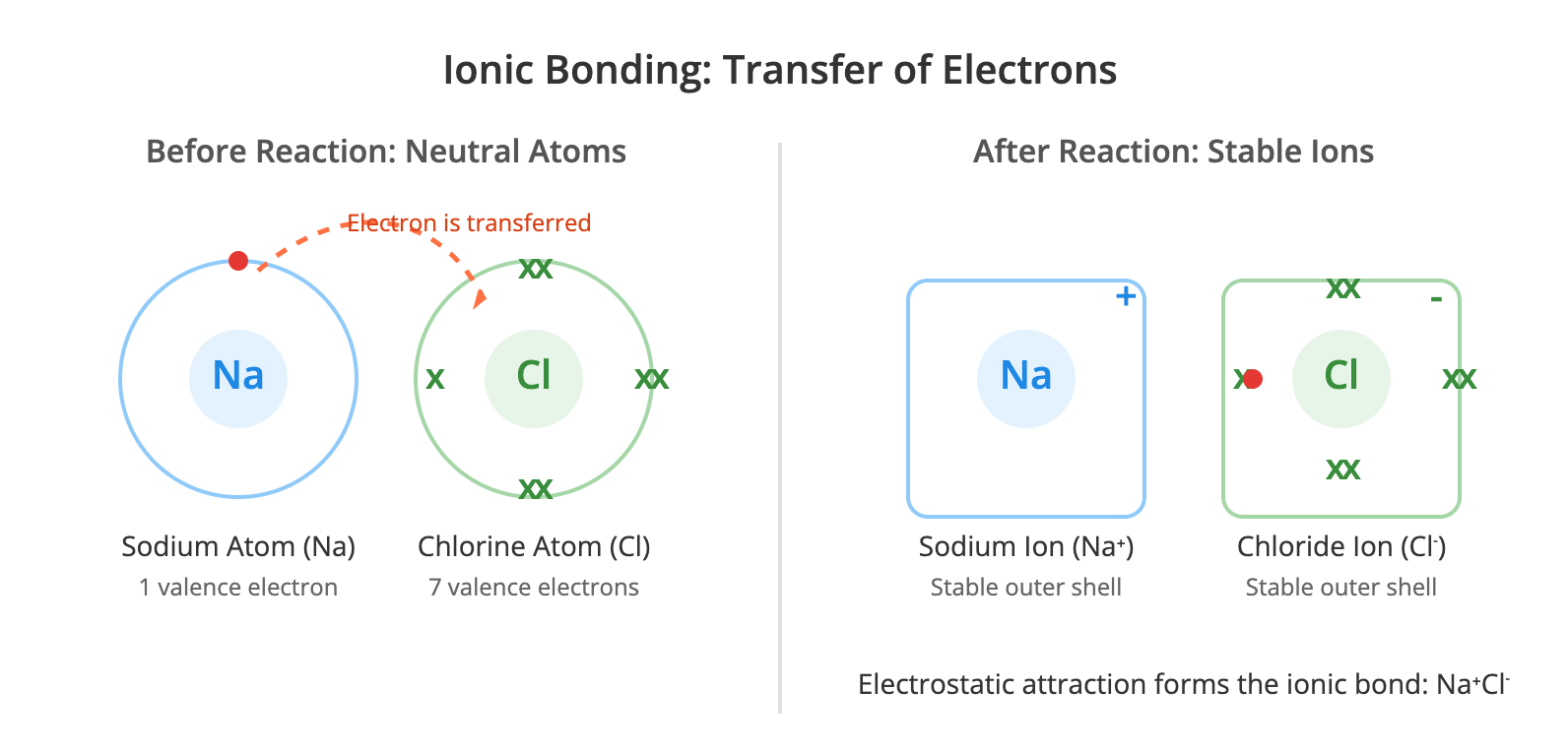
Download Ionic Bonding Ionic bonding is the electrostatic force of attraction between positively charged ions (cations) and negatively charged ions (anions), which are arranged in a giant crystal lattice. This type of bonding typically occurs between metals and non-metals. Metal atoms lose their outer shell electrons to form stable, positively charged ions, achieving the electronic configuration of a noble gas. Non-metal atoms gain these electrons to form stable, negatively charged ions, also achieving a noble gas configuration. The transfer of electrons is a key characteristic of ionic bond formation. For example, in sodium chloride (NaCl), a sodium atom transfers its
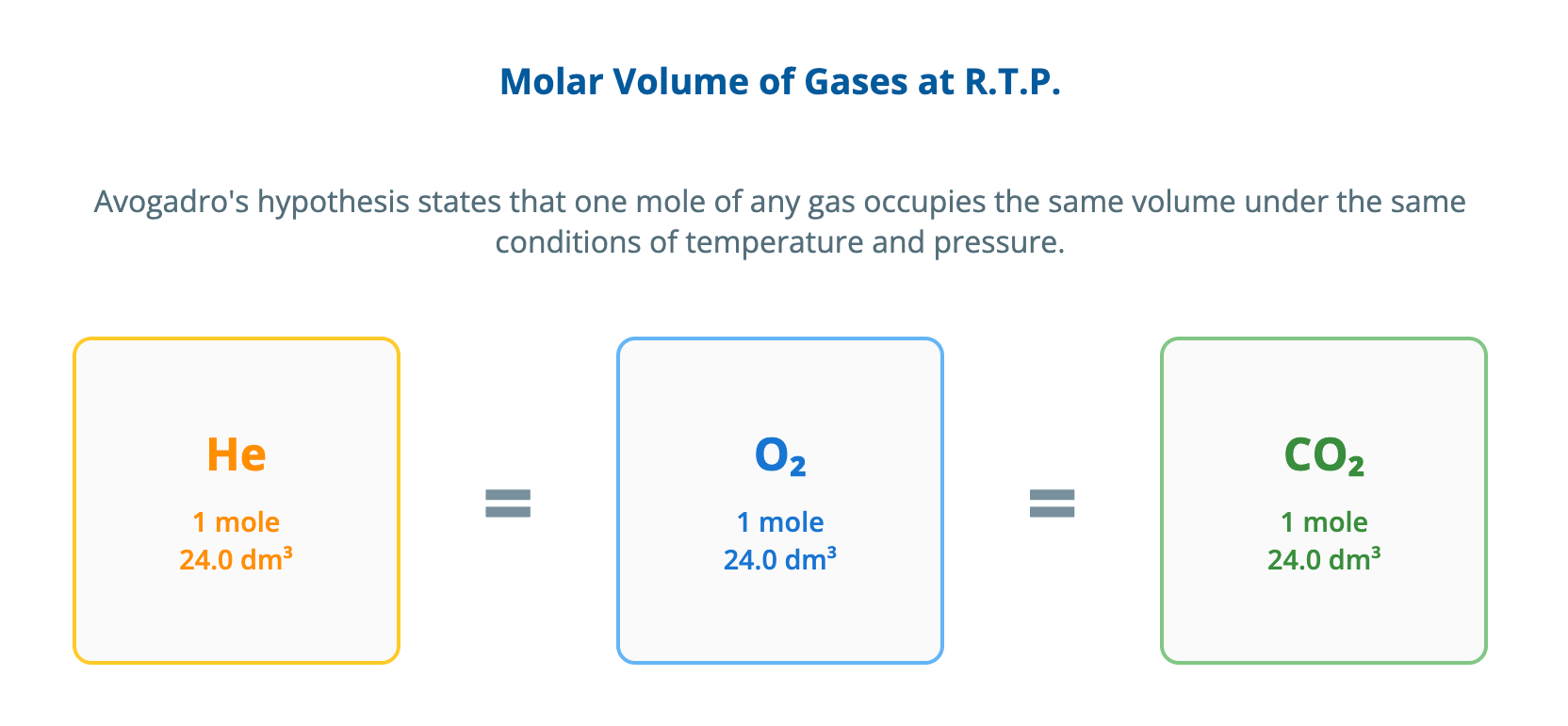
Calculations involving gas volumes notes | CIE | A-Level
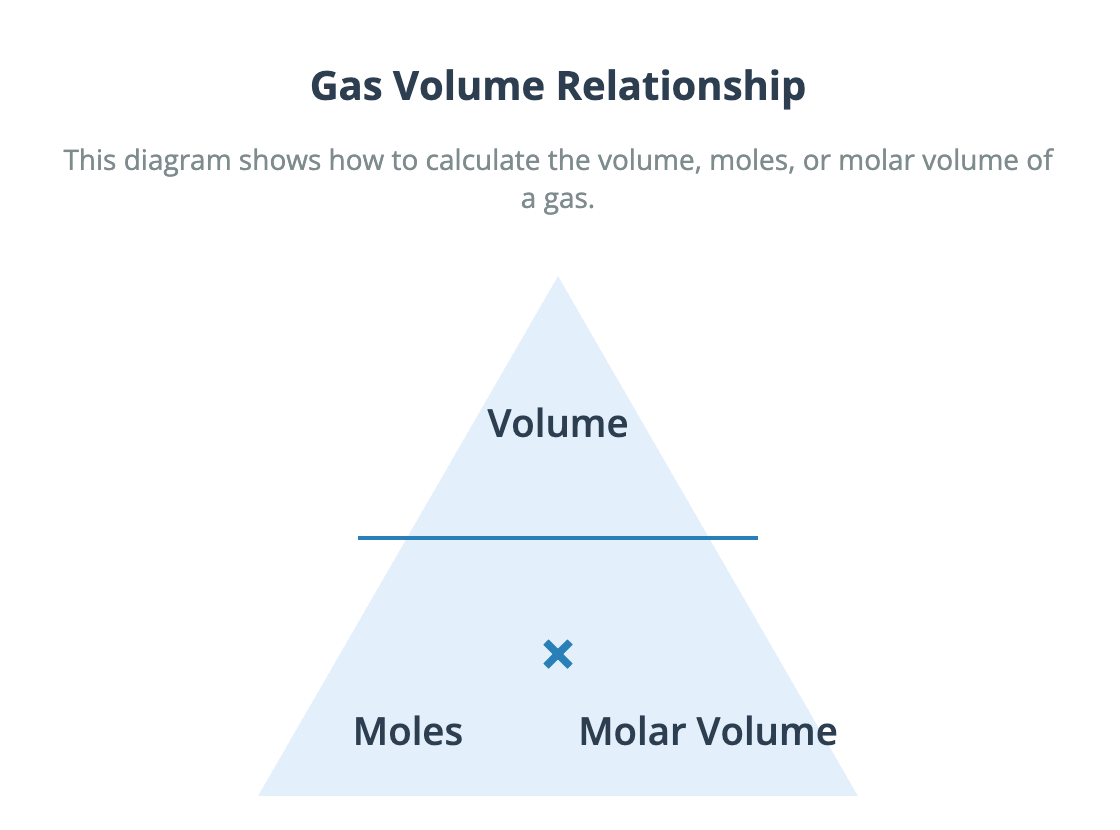
Download The Molar Volume of a Gas Avogadro's hypothesis states that equal volumes of any gas, measured under the same conditions of temperature and pressure, contain an equal number of molecules. This principle allows us to relate the volume of a gas directly to the number of moles present. The molar gas volume is the volume occupied by one mole of any gas. At room temperature and pressure (r.t.p.), which is defined as approximately 20 °C and 1 atmosphere, the molar gas volume is 24.0 dm³ mol⁻¹. This means that 24.0 dm³ of carbon dioxide, 24.0 dm³ of hydrogen, or
Solutions and Concentrations Revision Notes | CIE | A-Level
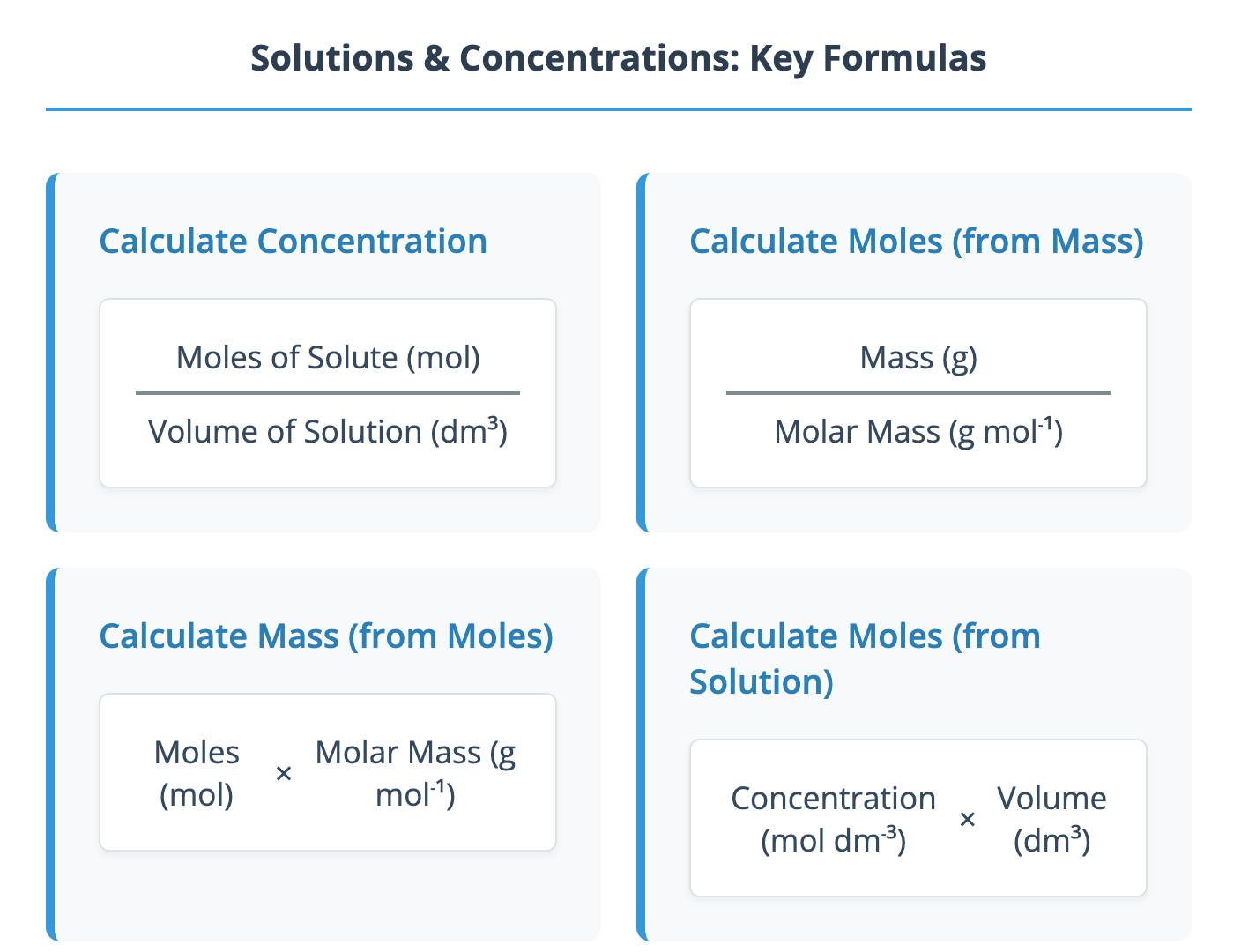
Download Defining Concentration The concentration of a solution is a measure of the amount of solute dissolved in a specific volume of solvent, which is typically water in A-Level Chemistry. The standard unit for concentration is moles per cubic decimetre (mol dm⁻³). It is important to remember the conversion between cubic centimetres (cm³) and cubic decimetres (dm³): 1 dm³ = 1000 cm³ A solution with a high amount of solute is described as concentrated, while one with a low amount of solute is described as dilute. The relationship between concentration, moles, and volume is given by the formula: Concentration (mol
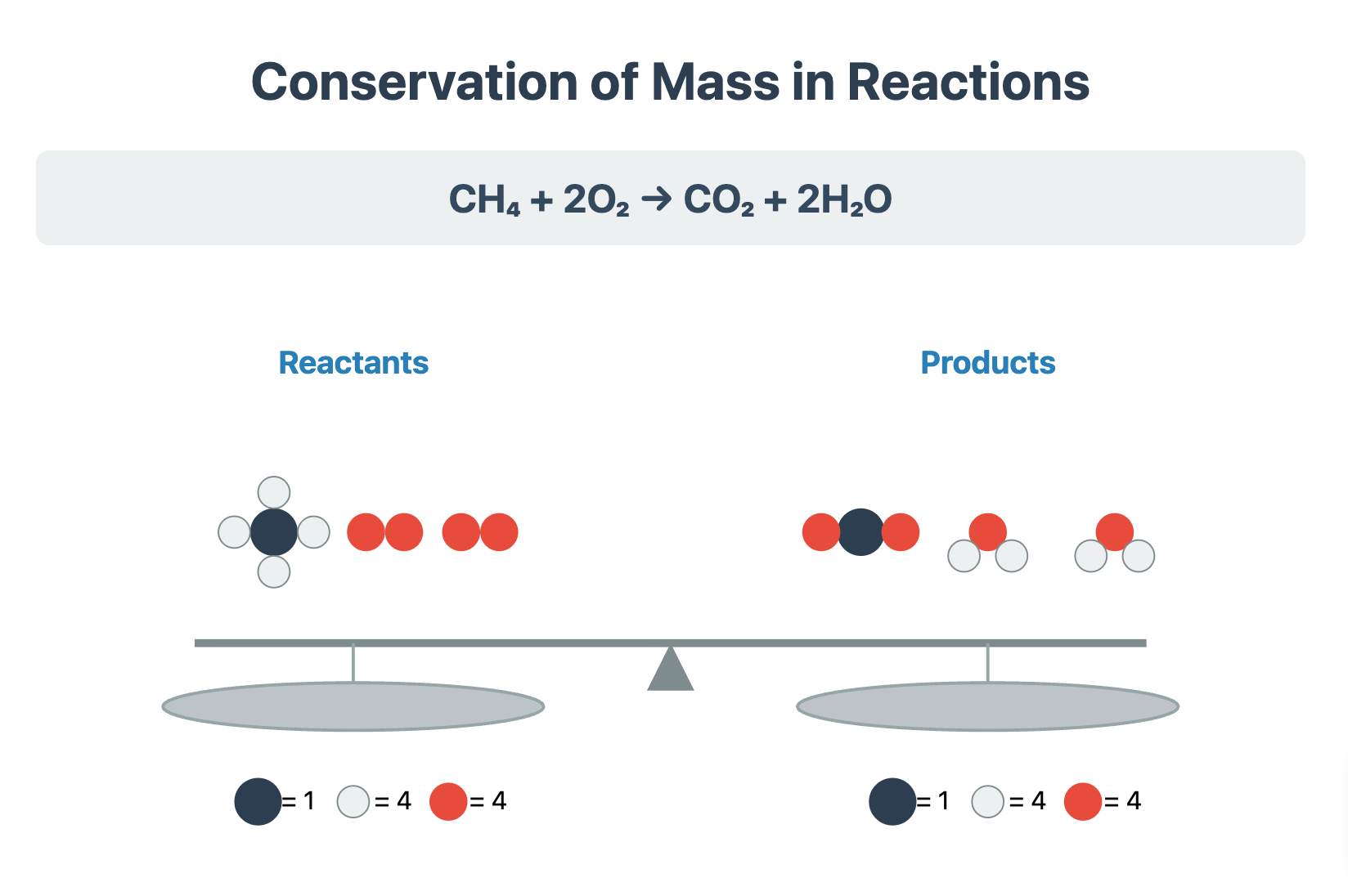
Balancing Chemical Equations Revision Notes | CIE | A-Level
Download The Principle of Balancing Equations A fundamental principle in chemistry is the law of conservation of mass, which states that atoms cannot be created or destroyed during a chemical reaction. Therefore, a chemical equation must be balanced to reflect this. A balanced chemical equation has the same number of each type of atom on both the reactants side and the products side. Writing a symbol equation is a shorthand method for describing a chemical reaction. It shows the number and type of atoms in the reactants and products. The process of balancing involves adjusting the stoichiometric numbers (the large
Ionic and Covalent Compounds Notes | CIE | A-Level Chemistry
Download Ionic Bonding Ionic bonding is the electrostatic force of attraction between oppositely charged ions within a giant ionic lattice structure. This type of bond typically forms between a metallic element and a non-metallic element. Formation of Ions The formation of ionic and covalent compounds is driven by the tendency of atoms to achieve a more stable electron configuration, usually that of the nearest noble gas. In ionic bonding, this is achieved through the transfer of one or more electrons from the outer shell of a metal atom to the outer shell of a non-metal atom. The metal atom loses
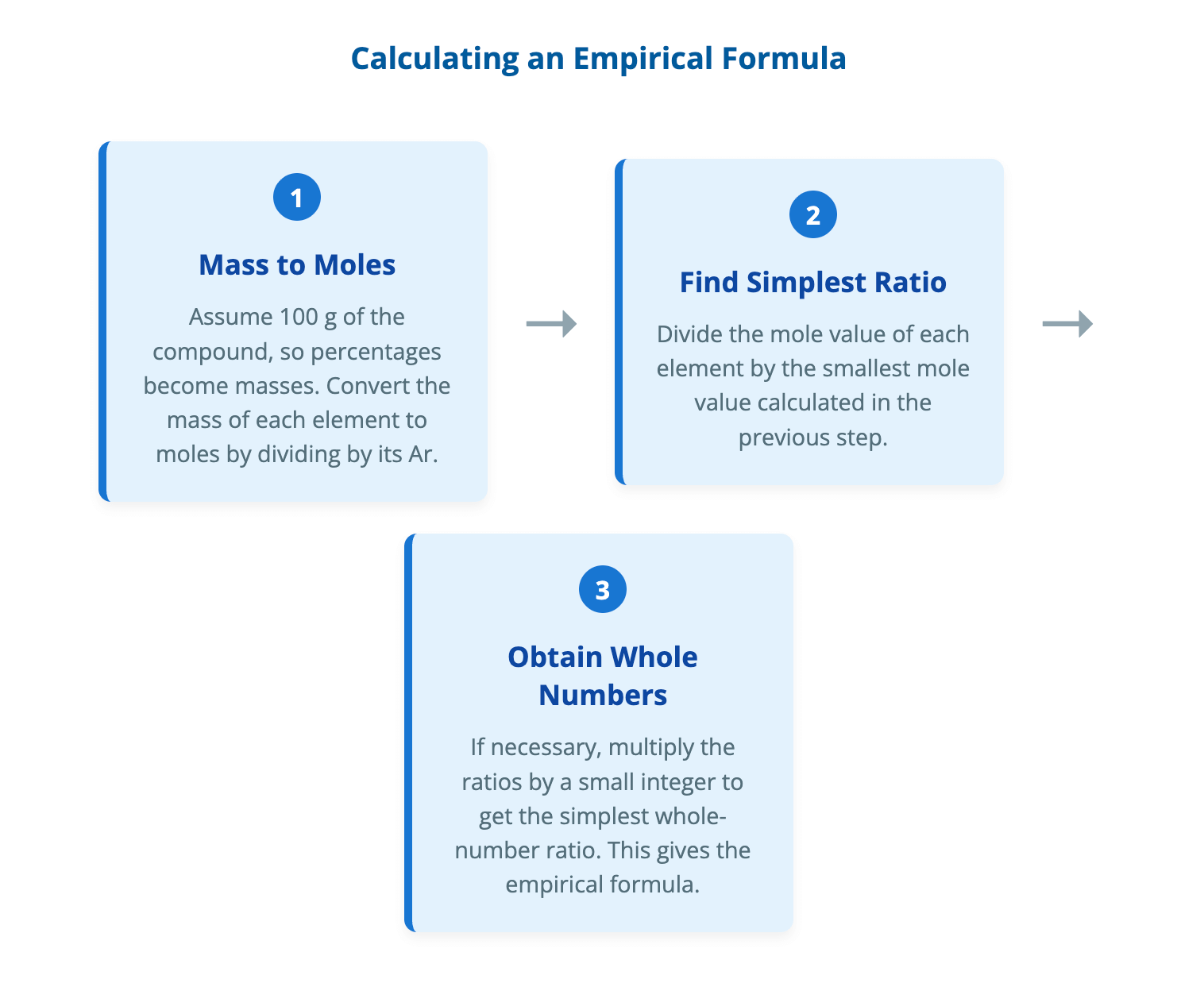
Empirical and Molecular Formulae Notes | CIE | A-Level Chemistry
Download The formula of a compound provides essential information about the elements it contains and the ratio in which they are present. There are two main types of chemical formulae used to describe the composition of a molecule: the empirical formula and the molecular formula. Defining Chemical Formulae Understanding the distinction between an empirical and a molecular formula is crucial for interpreting chemical information and solving stoichiometric problems. An empirical formula represents the simplest whole-number ratio of atoms of each element present in a compound. A molecular formula shows the actual number of atoms of each element in one molecule
Mole Concept and Stoichiometric Calculations Notes | CIE | A-Level
Download Stoichiometry is the study of the quantitative relationships between the amounts of reactants and products in a chemical reaction. The concept of the mole is central to all stoichiometric calculations. Relative Masses of Atoms and Molecules Relative Atomic Mass (Ar): The weighted average mass of an atom of an element, relative to one-twelfth of the mass of a carbon-12 atom. It is a ratio and has no units. Relative Molecular Mass (Mr): The sum of the relative atomic masses of all the atoms present in one molecule of a compound. For ionic compounds, this is referred to as the
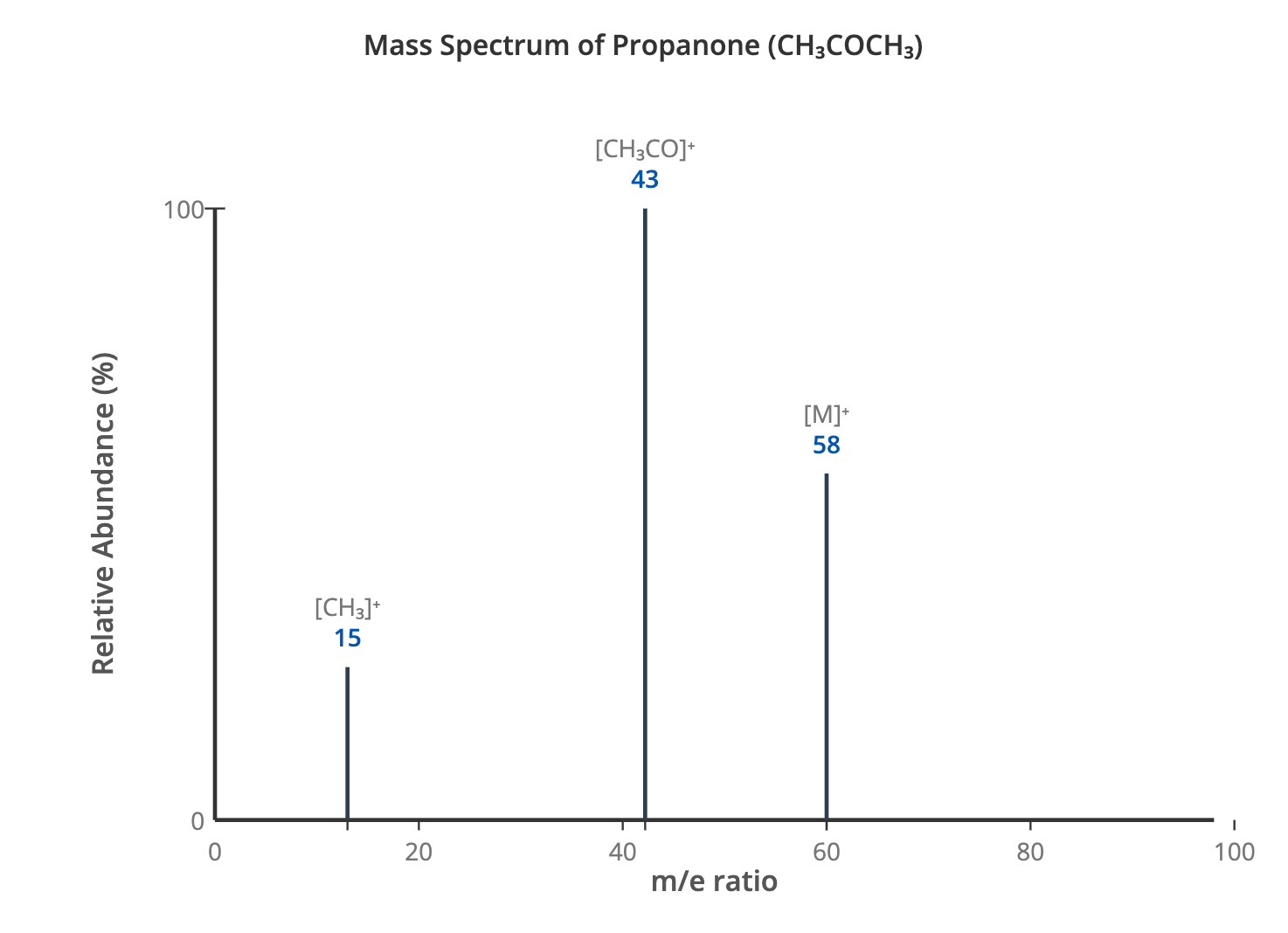
Mass Spectrometry Revision Notes | CIE | A-Level Chemistry
Download Principles of Mass Spectrometry Mass spectrometry is a powerful analytical technique used to measure the mass-to-charge ratio of ions. It can determine the relative atomic mass of an element and is widely used in the identification of organic compounds. The process involves several key stages: Vaporisation: The sample is heated and vaporised to produce gaseous atoms or molecules. Ionisation: The gaseous particles are bombarded with high-energy electrons, which knock out one or more electrons from each particle to form positive ions. Acceleration: The positive ions are accelerated by an electric field so that they all have the same kinetic
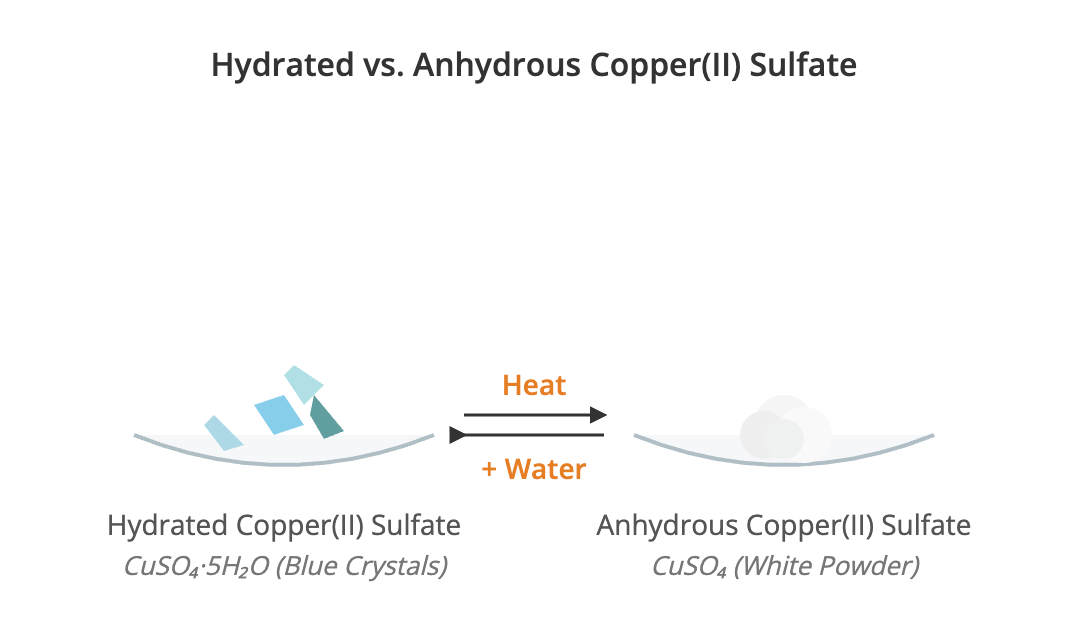
Hydrated and Anhydrous Compounds Revision Notes | CIE
Download Water of Crystallisation Some crystalline compounds incorporate a fixed number of water molecules into their structural lattice. This water is referred to as the water of crystallisation. Key Definitions A hydrated compound is a substance that contains water of crystallisation within its structure. A well-known example is hydrated copper(II) sulfate, CuSO₄·5H₂O, which is blue. An anhydrous compound is a substance that does not contain water of crystallisation. For example, anhydrous copper(II) sulfate, CuSO₄, is a white powder. A single compound can have different degrees of hydration. For instance, cobalt(II) chloride exists as both cobalt(II) chloride-6-water (CoCl₂·6H₂O) and cobalt(II) chloride-2-water