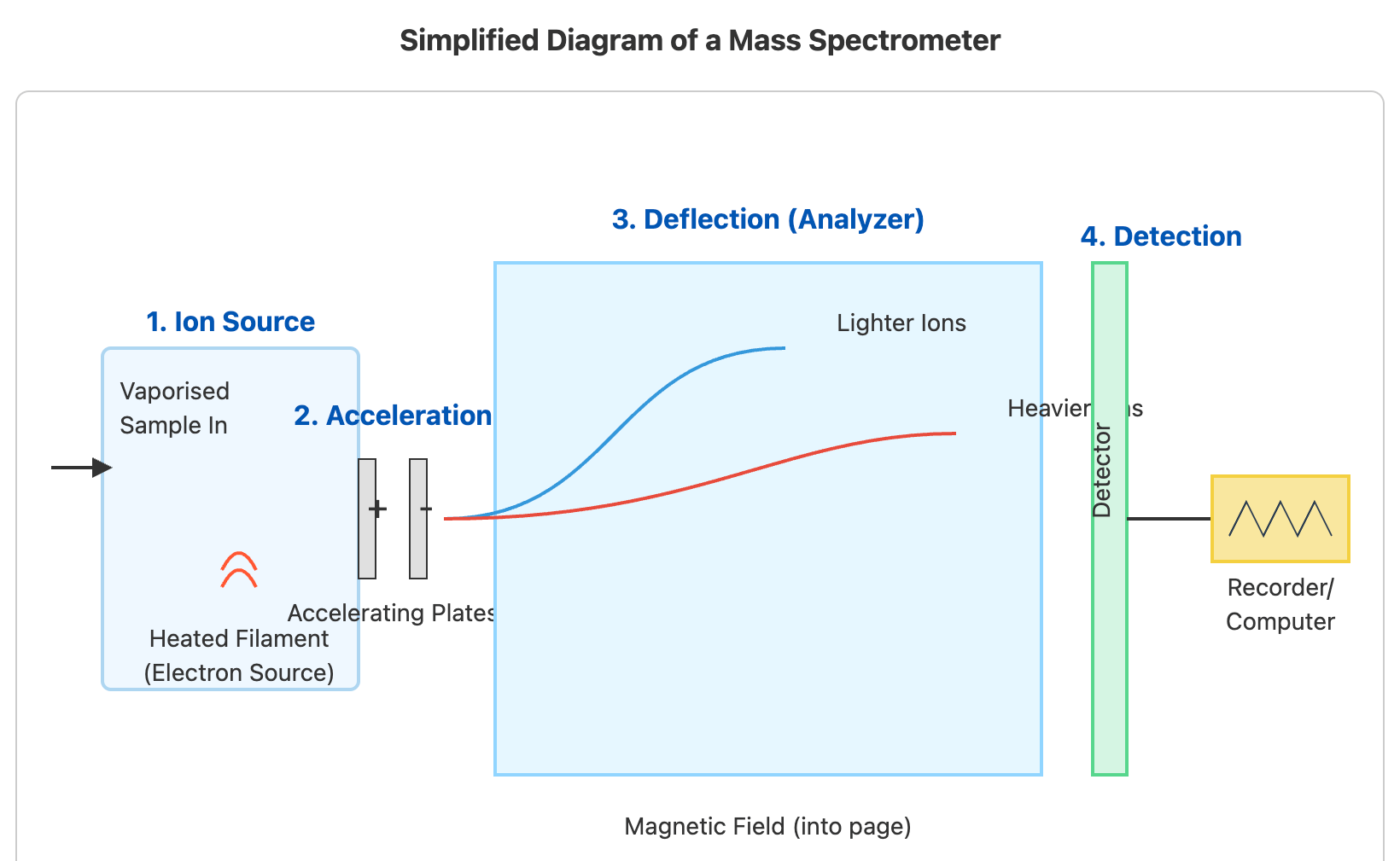
Relative Atomic Mass Revision Notes | CIE | A-Level Chemistry
Download Defining Atomic Mass To compare the masses of different atoms, chemists use a standard scale. All atomic masses are measured relative to a standard atom, which is an isotope of carbon, carbon-12. Unified Atomic Mass Unit (u) The unified atomic mass unit is the standard unit for atomic masses. It is defined as exactly one-twelfth of the mass of a single, unbound neutral atom of carbon-12 in its ground state. 1 u is approximately equal to 1.66 x 10⁻²⁷ kg. Relative Isotopic Mass This refers to the mass of an atom of a specific isotope of an element, measured
Isotopes Revision Notes | CIE | A-Level Chemistry
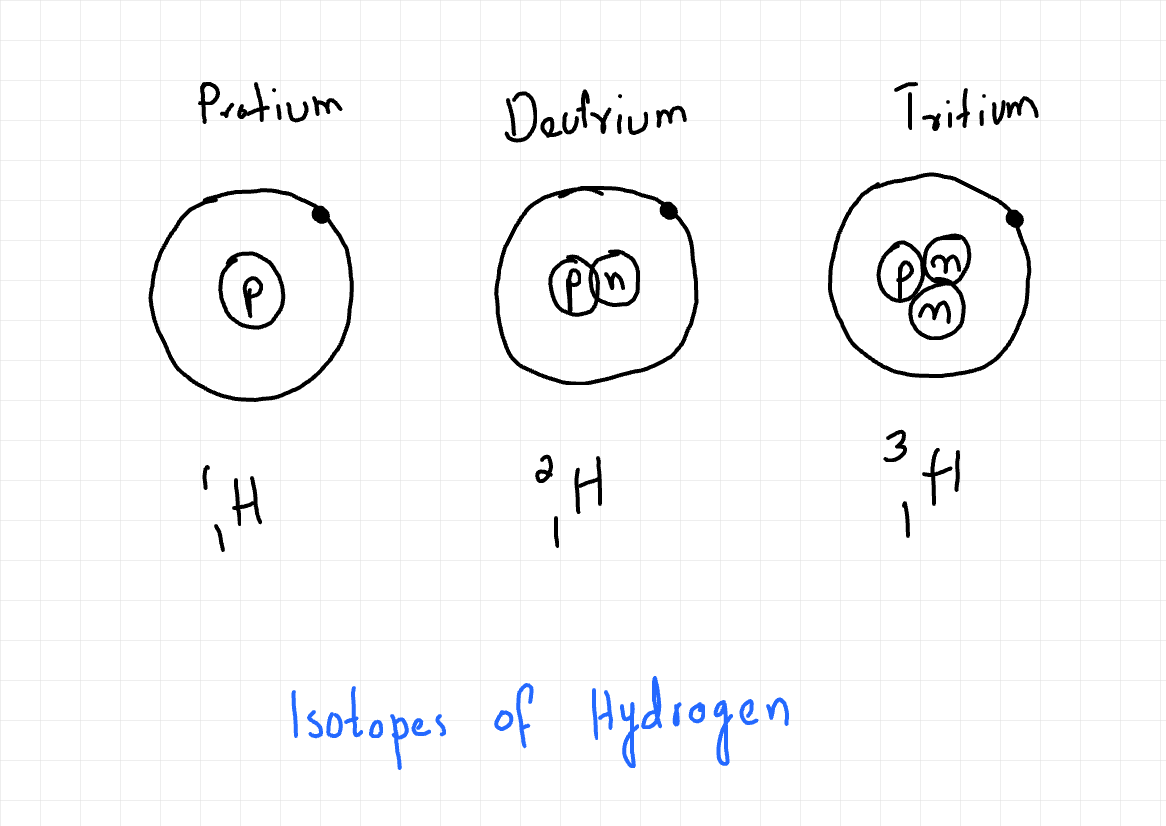
Download Defining Isotopes Isotopes are atoms of the same element that possess the same number of protons but a different number of neutrons. This means they share the same atomic (proton) number (Z) but have different mass (nucleon) numbers (A). Because isotopes of an element have the same number of protons, they also have the same number of electrons in a neutral atom. The identity of an element is determined solely by its atomic number. Isotopes are represented using the following notation: ᴬzX Where: X is the chemical symbol of the element. A is the mass (nucleon) number (total number
Subatomic Particles Revision Notes | CIE | A-Level Chemistry
Download An atom, once believed to be the smallest indivisible unit of matter, is fundamentally composed of three key types of subatomic particles: the proton, the neutron, and the electron. The arrangement and number of these particles within an atom determine the chemical identity and properties of an element. The vast majority of an atom's volume is empty space, with its mass concentrated in a tiny, dense central region called the nucleus. Properties of Subatomic Particles Each subatomic particle is defined by its relative mass, relative electrical charge, and location within the atom. For chemical purposes, we use relative values
Atomic Structure Revision Notes | CIE | A-Level Chapter 1
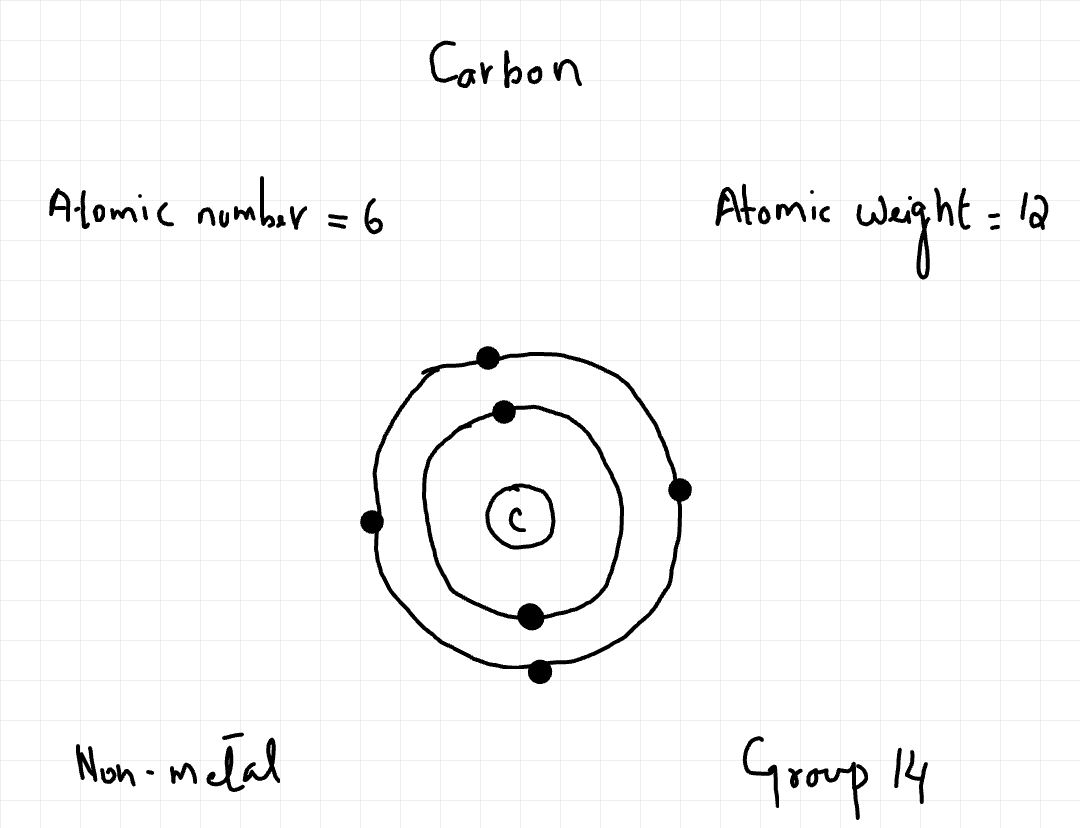
Download Inside the Atom The modern understanding of atomic structure is centered on a model where the atom is mostly empty space. At the center lies a very small, dense nucleus, which contains positively charged protons and neutral neutrons. Collectively, protons and neutrons are known as nucleons. The nucleus contains nearly all the mass of the atom. Surrounding the nucleus are negatively charged electrons. These electrons occupy specific energy levels, often referred to as electron shells. In a neutral atom, the number of electrons is equal to the number of protons, resulting in no overall charge. The properties of these
Ionization Energy Revision Notes | CIE | A-Level Chemistry
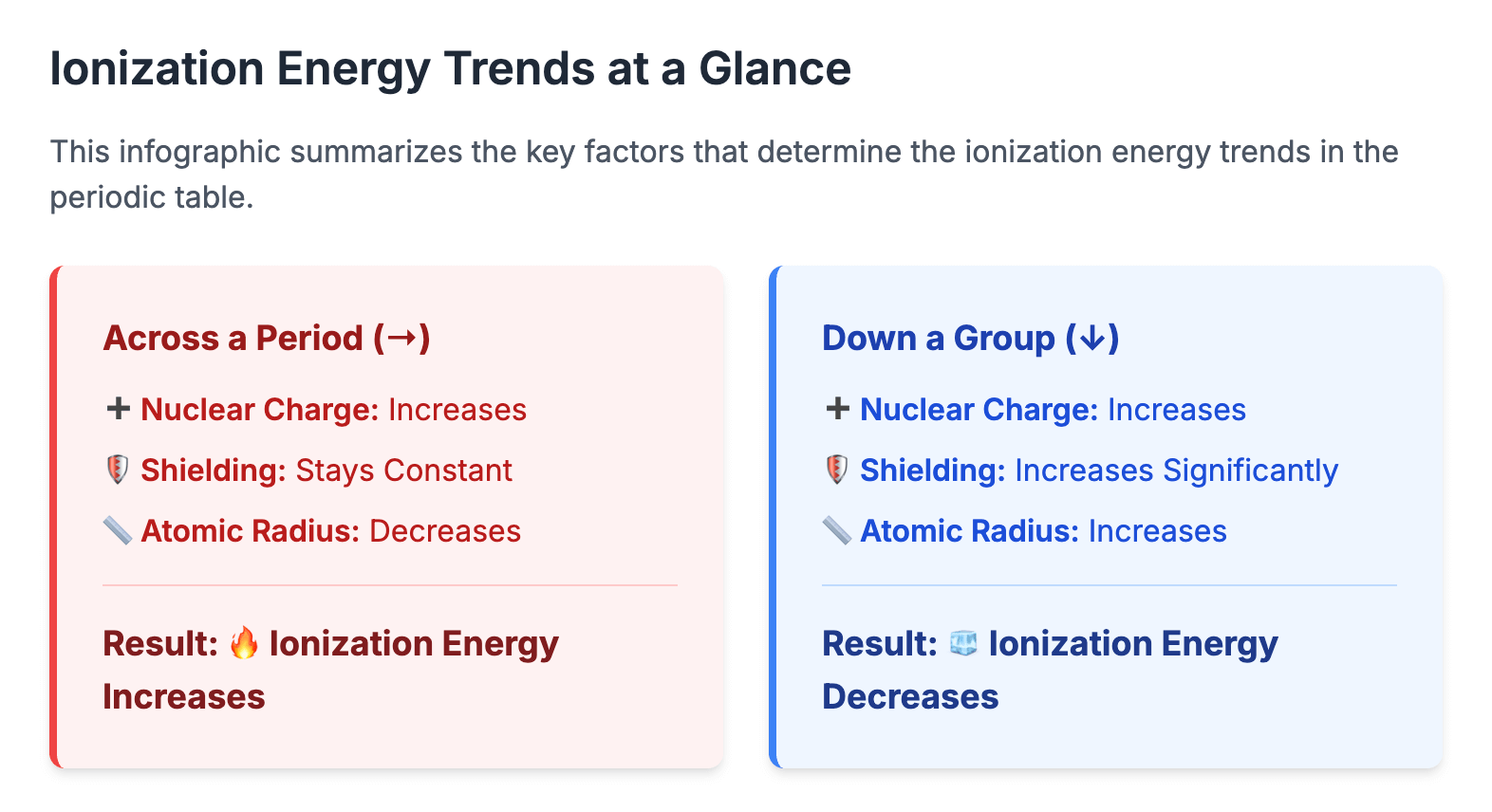
Download Ionization energy is a fundamental concept that provides strong evidence for the arrangement of electrons in shells and sub-shells within an atom. It measures the energy required to remove electrons from gaseous atoms or ions. First Ionization Energy The first ionization energy (IE₁) is defined as the energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions. The process can be represented by the general equation: X(g)→X+(g) + e− For example, for calcium: Ca(g)→Ca+(g)+e− IE1=+590 kJ mol⁻¹ It is an endothermic process, so ionization energy values
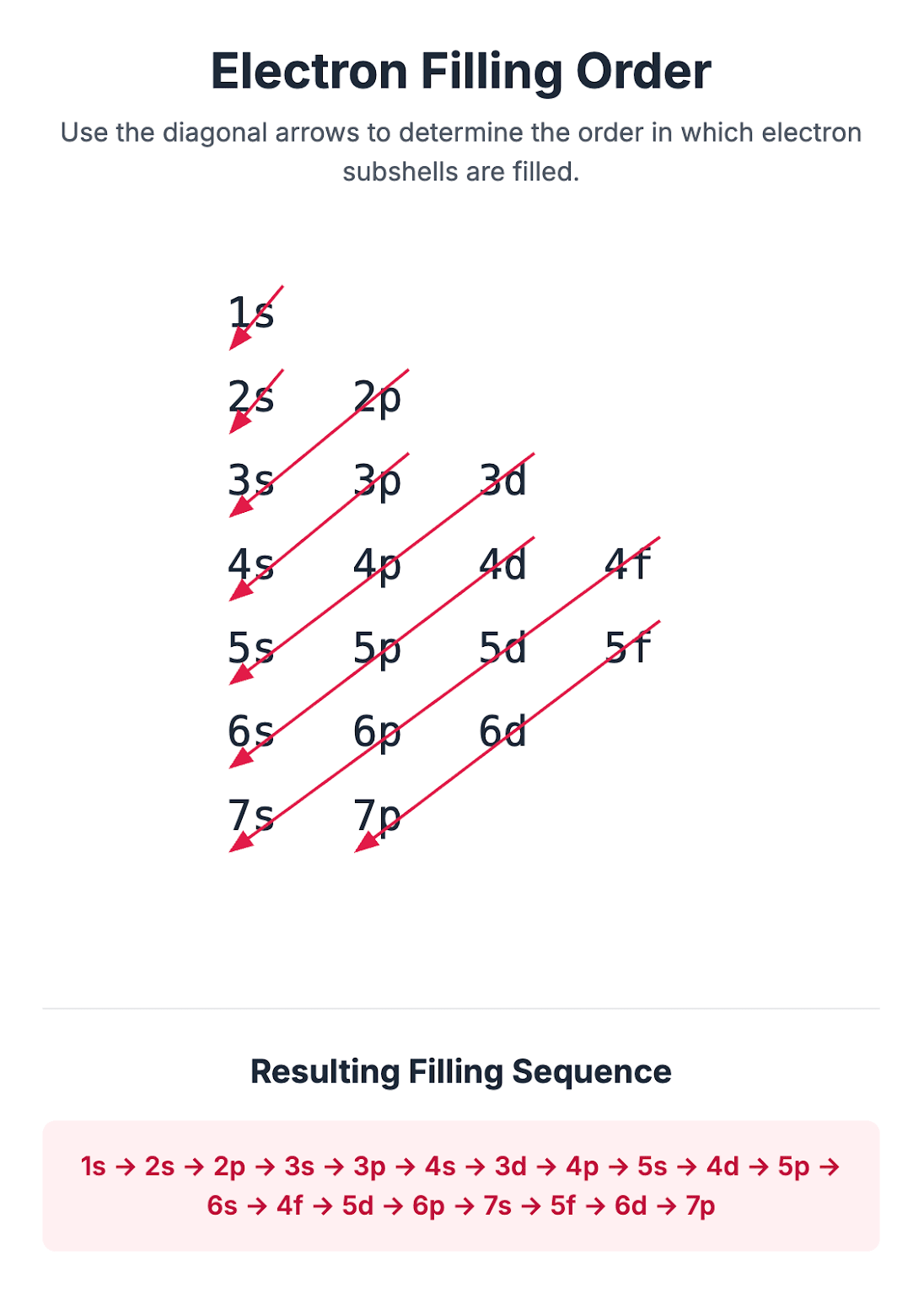
Subshells and Atomic Orbitals Revision Notes | CIE | A-Level
Download Quantum Subshells The principal quantum shells (energy levels) in an atom, except for the very first one, are split into subshells. These are identified by the letters s, p, and d. Each principal quantum shell contains a specific number of subshells, and within any given shell, the energy of the electrons increases in the order s < p < d. First principal shell (n=1): Contains one s subshell, holding a maximum of 2 electrons. Second principal shell (n=2): Contains an s subshell (2 electrons) and a p subshell (up to 6 electrons), for a total of 8 electrons. Third
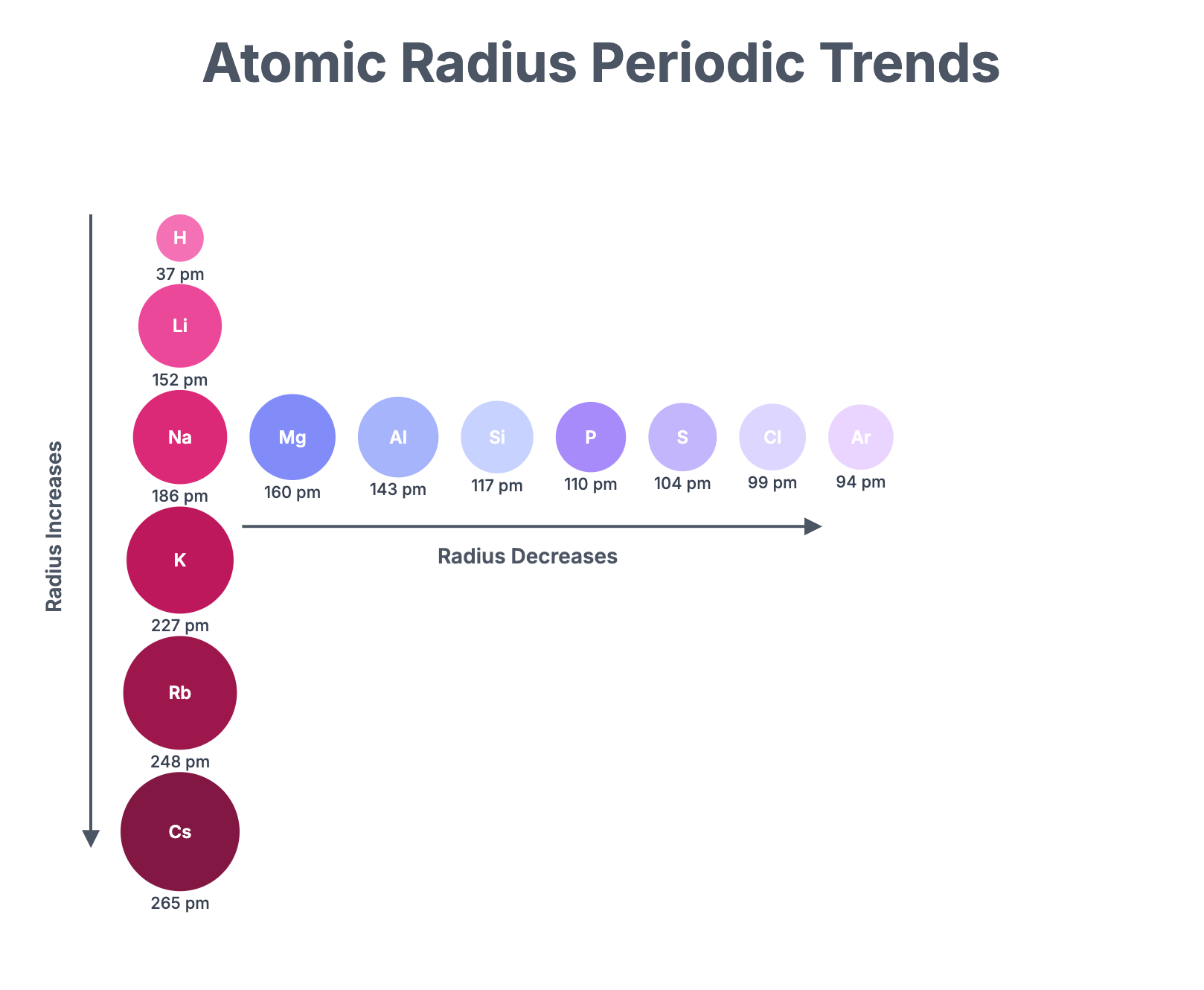
Atomic and Ionic Radii Revision Notes | CIE | A-Level
Download Atomic Radius The atomic radius of an element is a measure of the size of its atoms, typically the distance from the nucleus to the outermost electron shell. For elements that exist as diatomic molecules, this is often measured as half the distance between the nuclei of two covalently bonded atoms (the single covalent radius). Across a Period Across Period 3, from sodium to chlorine, the atomic radius decreases. Reason: As you move across the period, the number of protons in the nucleus increases by one for each successive element. This results in a greater nuclear charge. The extra
Electronic Configurations Revision Notes | CIE | A-Level Chemistry
Representing Electronic Configurations The electronic configuration of an atom shows how electrons are arranged in their shells, sub-shells, and orbitals. We use a specific notation to represent this arrangement. For example, the electronic configuration of a hydrogen atom is written as 1s¹. The first number (1) indicates the principal quantum number or shell. The letter (s) refers to the sub-shell. The superscript number (¹) shows the number of electrons in that sub-shell. For elements with many electrons, a shorthand notation is often used. The electronic configuration of the preceding noble gas is represented by its symbol in square brackets, followed