The Principle of Balancing Equations
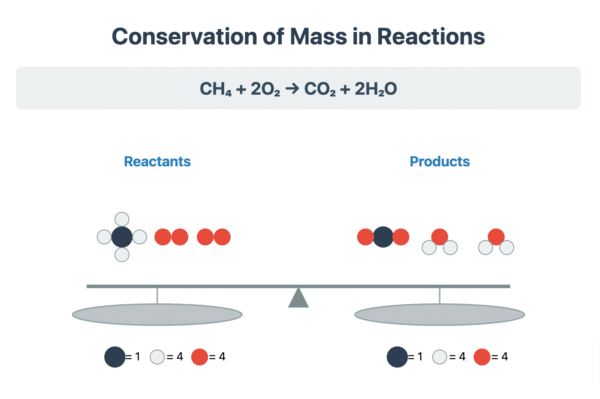
A fundamental principle in chemistry is the law of conservation of mass, which states that atoms cannot be created or destroyed during a chemical reaction. Therefore, a chemical equation must be balanced to reflect this. A balanced chemical equation has the same number of each type of atom on both the reactants side and the products side.
Writing a symbol equation is a shorthand method for describing a chemical reaction. It shows the number and type of atoms in the reactants and products. The process of balancing involves adjusting the stoichiometric numbers (the large numbers in front of chemical formulae) until the atom count for each element is equal on both sides of the arrow.
A Step-by-Step Guide to Balancing
To balance a chemical equation, you should follow a systematic approach. It is crucial never to change the chemical formulae of the substances involved.
1. Write down the correct chemical formulae for all reactants and products.
Example: The reaction of hydrogen and oxygen to form water.
H₂ + O₂ → H₂O
2. Count the number of atoms of each element on both sides of the equation.
Reactants: 2 Hydrogen, 2 Oxygen
Products: 2 Hydrogen, 1 Oxygen
The oxygen atoms are unbalanced.
3. Balance one element at a time by placing stoichiometric numbers in front of the formulae. To balance the oxygen, place a ‘2’ in front of H₂O.
H₂ + O₂ → 2H₂O
4. Recount the atoms.
Reactants: 2 Hydrogen, 2 Oxygen
Products: 4 Hydrogen, 2 Oxygen
The oxygen is now balanced, but the hydrogen is not.
5. Continue balancing until all elements are equal. To balance the hydrogen, place a ‘2’ in front of H₂.
2H₂ + O₂ → 2H₂O
6. Perform a final count to verify.
Reactants: 4 Hydrogen, 2 Oxygen
Products: 4 Hydrogen, 2 Oxygen
The equation is now balanced.
When balancing combustion reactions of organic compounds, it is often easiest to balance the carbon atoms first, then the hydrogen atoms, and leave the oxygen atoms until last.
Using State Symbols
To provide more detail, chemical equations should include state symbols, which indicate the physical state of each substance.
- (s) for solid
- (l) for liquid
- (g) for gas
- (aq) for aqueous (dissolved in water)
Example: The reaction between solid calcium carbonate and aqueous hydrochloric acid.
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + CO₂(g) + H₂O(l)
Ionic Equations
Many reactions occur in aqueous solutions where ionic compounds are dissociated into their constituent ions. In these reactions, some ions do not take part in the chemical change. These are called spectator ions. An ionic equation simplifies the overall chemical equation by showing only the particles that actually react, omitting the spectator ions.
To write an ionic equation:
1. Start with the full, balanced chemical equation.
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
2. Rewrite the equation showing the ions for all aqueous substances. Do not separate solids, liquids, or gases into ions.
Zn(s) + Cu²⁺(aq) + SO₄²⁻(aq) → Zn²⁺(aq) + SO₄²⁻(aq) + Cu(s)
3. Identify and cancel out the spectator ions, which are identical on both sides. In this case, the sulfate ion (SO₄²⁻) is the spectator.
4. Write the final ionic equation.
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
5. An ionic equation must be balanced for both atoms and charges. For precipitation reactions, a simplified method is to write the formula of the precipitate as the product and the ions that form it as the reactants.
Example: The precipitation of iron(II) hydroxide.
Fe²⁺(aq) + 2OH⁻(aq) → Fe(OH)₂(s)
Exam-Style Questions and Answers
Question 1
Balance the following chemical equation for the reaction of iron(III) oxide with carbon monoxide to form iron and carbon dioxide.
…Fe₂O₃(s) + …CO(g) → …Fe(s) + …CO₂(g)
Answer
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
Verification:
Reactants: 2 Fe, (3+3) = 6 O, 3 C
Products: 2 Fe, (3*2) = 6 O, 3 C
The atoms of each element are balanced on both sides.
Question 2
Write a balanced chemical equation, including state symbols, for the complete combustion of liquid propane (C₃H₈).
Answer
C₃H₈(l) + 5O₂(g) → 3CO₂(g) + 4H₂O(l)
Explanation:
- Balance Carbon: There are 3 C atoms on the left, so 3 CO₂ are needed on the right.
- Balance Hydrogen: There are 8 H atoms on the left, so 4 H₂O are needed on the right.
- Balance Oxygen: There are now (3 * 2) + 4 = 10 O atoms on the right, so 5 O₂ are needed on the left.
- Add state symbols.
Question 3
An aqueous solution of silver nitrate (AgNO₃) reacts with an aqueous solution of magnesium chloride (MgCl₂) to form a solid precipitate of silver chloride (AgCl) and an aqueous solution of magnesium nitrate (Mg(NO₃)₂). Write the balanced ionic equation for this reaction.
Answer
2Ag⁺(aq) + 2Cl⁻(aq) → 2AgCl(s)
Which simplifies to:
Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
Explanation:
Full balanced equation: 2AgNO₃(aq) + MgCl₂(aq) → 2AgCl(s) + Mg(NO₃)₂(aq)
Full ionic equation: 2Ag⁺(aq) + 2NO₃⁻(aq) + Mg²⁺(aq) + 2Cl⁻(aq) → 2AgCl(s) + Mg²⁺(aq) + 2NO₃⁻(aq)
Spectator ions (unchanged on both sides) are Mg²⁺(aq) and NO₃⁻(aq).
Removing spectator ions gives the net ionic equation: 2Ag⁺(aq) + 2Cl⁻(aq) → 2AgCl(s).
This is simplified to the lowest whole number ratio: Ag⁺(aq) + Cl⁻(aq) → AgCl(s).
