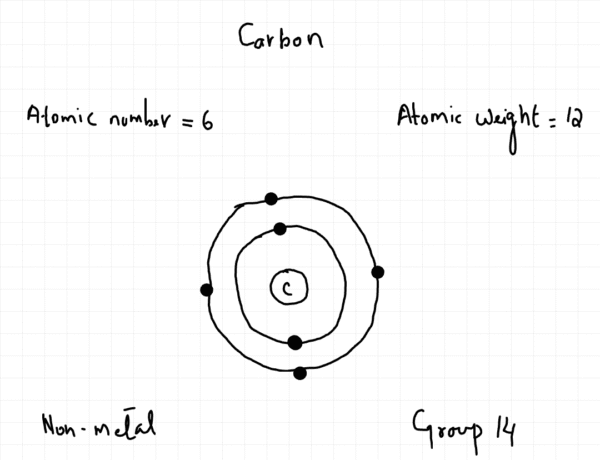
Inside the Atom
The modern understanding of atomic structure is centered on a model where the atom is mostly empty space. At the center lies a very small, dense nucleus, which contains positively charged protons and neutral neutrons. Collectively, protons and neutrons are known as nucleons. The nucleus contains nearly all the mass of the atom.
Surrounding the nucleus are negatively charged electrons. These electrons occupy specific energy levels, often referred to as electron shells. In a neutral atom, the number of electrons is equal to the number of protons, resulting in no overall charge.
The properties of these subatomic particles are compared using relative values:
- Proton: Relative mass of 1, Relative charge of +1
- Neutron: Relative mass of 1, Relative charge of 0
- Electron: Relative mass of approximately 1/1836, Relative charge of -1
When beams of these particles travel through an electric field, their behavior is determined by their charge. Protons are deflected towards the negative plate, electrons are deflected towards the positive plate (and show a greater deflection than protons due to their much smaller mass), and neutrons are not deflected at all.
Atomic and Mass Numbers
The identity of an element is defined by its atomic number (Z), which is the number of protons in the nucleus of its atoms. Every atom of a specific element has the same atomic number.
The mass number (A), also known as the nucleon number, is the total count of protons and neutrons in the nucleus.
From these two numbers, the quantity of each subatomic particle in a neutral atom can be determined:
- Number of protons = Z
- Number of electrons = Z
- Number of neutrons = A – Z
When an atom gains or loses electrons, it becomes an ion. A positive ion (cation) is formed by losing electrons, and a negative ion (anion) is formed by gaining electrons. The number of protons and neutrons remains unchanged. For an ion, the number of electrons is Z – (charge). For example, a Mg²⁺ ion (Z=12) has 12 – (+2) = 10 electrons.
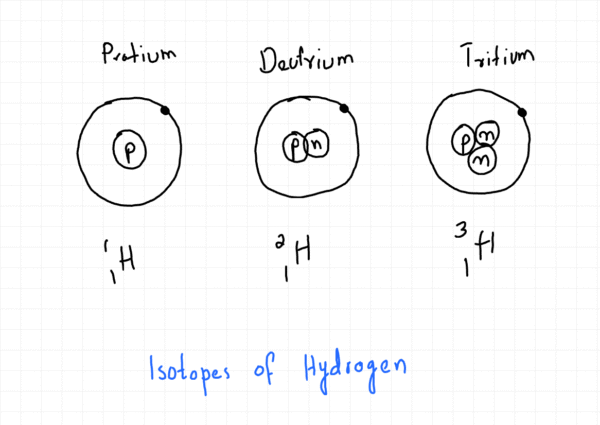
Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means isotopes of an element share the same atomic number (Z) but have different mass numbers (A).
A specific isotope is represented using the notation ᴬZX, where X is the element’s symbol. For example, the two main isotopes of chlorine are ³⁵₁₇Cl and ³⁷₁₇Cl.
Because isotopes of an element have the same number of electrons and the same electron configuration, they share the same chemical properties. Their reactions are identical. However, they exhibit different physical properties, such as mass and density, due to the difference in the number of neutrons in their nuclei. For instance, hydrogen-2 (deuterium) is denser than hydrogen-1 (protium).
Exam-Style Questions and Solutions
Question 1
Boron has two main isotopes, ¹⁰B and ¹¹B.
a. Deduce the number of i) protons, ii) neutrons, and iii) electrons in one neutral atom of the isotope ¹¹B.
b. What do you understand by the term isotope?
c. State the relative masses and charges of an electron, a neutron, and a proton.
Solution:
a. For the isotope ¹¹B (Boron-11):
* The atomic number (Z) of Boron is 5.
* i) Protons: The number of protons is equal to the atomic number. So, there are 5 protons.
* iii) Electrons: In a neutral atom, the number of electrons equals the number of protons. So, there are 5 electrons.
* ii) Neutrons: The number of neutrons is the mass number (A) minus the atomic number (Z). So, 11 – 5 = 6 neutrons.
b. Isotopes are atoms of the same element (having the same number of protons) but with different numbers of neutrons, and therefore different mass numbers.
c.
* Electron: Relative mass ≈ 1/1836, Relative charge = -1
* Neutron: Relative mass = 1, Relative charge = 0
* Proton: Relative mass = 1, Relative charge = +1
Question 2
The symbols below describe two isotopes of the element uranium.
²³⁵U and ²³⁸U
a. State the meaning of the term isotope.
b. State two ways in which these two isotopes of uranium are identical.
c. State how these isotopes differ.
d. State the number of electrons present in one U²⁺ ion.
Solution:
a. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons.
b. The two isotopes are identical in:
1. Number of protons (92 protons for uranium).
2. Number of electrons in a neutral atom (92 electrons), which gives them identical chemical properties.
c. The isotopes differ in:
1. Number of neutrons (235 – 92 = 143 neutrons in U-235; 238 – 92 = 146 neutrons in U-238).
2. Mass number (235 vs 238).
3. Physical properties like mass and density.
d. The atomic number of uranium (U) is 92. A neutral uranium atom has 92 electrons. A U²⁺ ion has lost two electrons.
* Number of electrons = 92 – 2 = 90 electrons.
Question 3
a. Describe the structure of an atom, giving details of the subatomic particles present.
b. Copy and complete the table:
| Neutral atom | Atomic number | Nucleon number | Numbers of each subatomic particle present |
| Mg | 12 | 24 | |
| Al | 13 | 27 |
c. Explain why atoms are neutral.
Solution:
a. An atom consists of a small, dense, positively charged nucleus at its center, which contains protons (relative charge +1) and neutrons (relative charge 0). The nucleus accounts for almost all the atom’s mass. Surrounding the nucleus are electrons (relative charge -1), which are much lighter and occupy regions of space called electron shells or energy levels. The majority of the atom is empty space.
b. Completed table:
| Neutral atom | Atomic number | Nucleon number | Numbers of each subatomic particle present |
| Mg | 12 | 24 | 12 protons, 12 neutrons, 12 electrons |
| Al | 13 | 27 | 13 protons, 14 neutrons, 13 electrons |
Calculation for Mg: Protons = 12, Electrons = 12, Neutrons = 24 – 12 = 12.
Calculation for Al: Protons = 13, Electrons = 13, Neutrons = 27 – 13 = 14.
c. Atoms are neutral because they contain an equal number of positively charged protons in the nucleus and negatively charged electrons surrounding the nucleus. The equal and opposite charges cancel each other out, resulting in no overall charge.
