Atomic Radius
The atomic radius of an element is a measure of the size of its atoms, typically the distance from the nucleus to the outermost electron shell. For elements that exist as diatomic molecules, this is often measured as half the distance between the nuclei of two covalently bonded atoms (the single covalent radius).
Across a Period
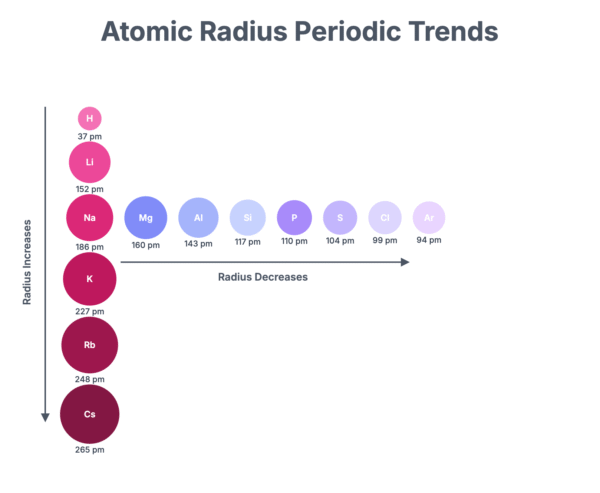
Across Period 3, from sodium to chlorine, the atomic radius decreases.
Reason:
- As you move across the period, the number of protons in the nucleus increases by one for each successive element. This results in a greater nuclear charge.
- The extra electron added for each element enters the same principal energy level (the third shell).
- The effect of electron shielding from the inner shells remains relatively constant.
- The stronger attraction from the increasing nuclear charge pulls the electrons in the outer shell closer to the nucleus, thus reducing the atomic radius.
Down a Group
Down a group in the Periodic Table, the atomic radius increases.
Reason:
- Each element down the group has one more principal electron shell than the one above it.
- Although the nuclear charge increases, the effect of the additional shell is more significant. The outermost electrons are further from the nucleus.
- Additionally, the inner electron shells provide a shielding effect, which reduces the pull of the nucleus on the outer electrons.
- This combination of increased distance and shielding outweighs the increased nuclear charge, leading to a larger atomic radius.
Ionic Radius
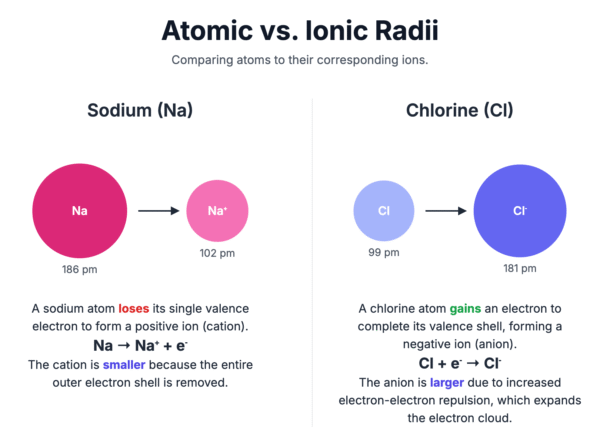
The ionic radius is the measure of an ion’s size in a crystal lattice. The size of an ion is different from the size of its parent atom.
- Positive ions (cations) are smaller than their corresponding atoms. This is because they have lost their outer shell of electrons, and the remaining electrons are pulled more strongly by the unchanged positive nuclear charge.
- Negative ions (anions) are larger than their corresponding atoms. This is because the atom has gained one or more electrons into its outer shell, increasing the repulsion between electrons and causing the electron cloud to expand.
Across a Period
The trend in ionic radius across a period is not continuous due to the change from positive to negative ions.
- For the positive ions (e.g., Na⁺, Mg²⁺, Al³⁺), the ionic radius decreases. The nuclear charge increases while the number of electrons remains the same, pulling the electron shells closer.
- For the negative ions (e.g., P³⁻, S²⁻, Cl⁻), the ionic radius also decreases. As the nuclear charge increases, the electrons are pulled more tightly towards the nucleus.
- There is a large increase in radius when moving from the positive ions to the negative ions within the same period because the anions have an additional electron shell compared to the cations.
Down a Group
Down a group, the ionic radius increases for ions with the same charge (e.g., Li⁺, Na⁺, K⁺).
Reason: Similar to the trend in atomic radii, each successive ion has an additional electron shell. This increased distance from the nucleus is the primary factor, causing the ionic size to increase despite the higher nuclear charge.
